Abstract
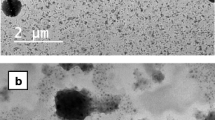
The purpose of this study was to prepare chitosan-dextran sulphate and chitosan-sodium tripolyphosphate nanoparticles of Dorzolamide HCl. Dorzolamide HCl is used in higher doses (2–2.2%) topically resulting topical as well as systemic side effects, reducing the dose and making the formulation stay in ocular cavity can reduce the side effects. In the present study mucoadhesive nanoformulations of Dorzolamide HCl have been prepared by Ionotropic gelation mechanism and characterized for different parameters like particle size, zeta potential, drug entrapment, particle morphology; in-vitro drug release, ex-vivo transcorneal permeation and in vivo efficacy. The particles showed sustained in vitro drug release which followed the Higuchi kinetic model and release the drug by a combination of dissolution and diffusion. The formulations, namely DD3 and DS6, having least particle size (182.63 ± 4.64 and 172.3 ± 9.03 nm) and highest zeta potential (43.03 ± 0.51 and 36.46 ± 0.59 mV) were selected for further studies. The particles were spherical and scattered. PXRD and DSC indicated decrease in crystallinity of drug in the nanoparticle formulation. Optimized formulations exhibited excellent transcorneal permeation with no signs of corneal damage (% hydration 75–80%) and found mucoadhesive (93.37 ± 1.86% and 91.59 ± 1.48%) and stable. The in vivo ocular hypotensive activity revealed significantly higher hypotensive activity (p < 0.05) with no signs of ocular irritation. Hence can be considered for the clinical application.






Similar content being viewed by others
References
Ameeduzzafar et al (2014) Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int J Biol Macromol 65:479–491
Berthold A, Cremer K, Kreuter J (1996) Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for antiinflammatory drugs. J Control Rel 39:17–25
Bhatta RS et al (2012) Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int J Pharm 432:105–112
Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–132
Cegnar M, Kos J, Kristl J (2004) Cystatin Incorporated in poly(lactide-co-glycolide) Nanoparticles: development and fundamental studies on preservation of its activity. Eur J Pharm Sci 22:357–364
Chen Y, VJ Mohanraj, F Wang, HAE Benson (2007) Designing chitosan-dextran sulfate nanoparticles using charge ratios. AAPS Pharmsci 8:E98
Das S, PK Suresh, R. Desmukh (2010) Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine 6:318–323
El-Gazayerly ON, Hikal AH (1997) Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int J Pharm 158:121–127
EP O’Donoghue et al (2000) A comparison of latanoprost and dorzolamide in patients with glaucoma and ocular hypertension: a 3 month, randomised study. Ireland latanoprost study group. Brit J Ophthalmol 84:579–582
Hasan A (2012) Formulation and evaluation of dorzolamide hydrochloride-loaded nanoparticles as controlled release drug delivery system. Asian J Pharm 6:67–73
Jõhannesson G et al (2014) Kinetics of γ-cyclodextrin nanoparticle suspension eye drops in tear fluid. Acta Ophthalmol 92:550–556
Katara R, DK Majumdar (2013) Colloids and surfaces: biointerfaces eudragit RL 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloid Surf B 103:455–462
Katiyar S et al (2014) In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohyd Polym 102:117–124
Kaur IP, Smitha R, D Aggarwal, M Kapil (2002) Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm 248:1–14
Koocheki S, Madaeni SS, Niroomandi P (2011) Development of an enhanced formulation for delivering sustained release of buprenorphine hydrochloride. Saudi Pharm J 19:255–262
Makino K et al (2000) Pulsatile drug release from poly (lactide-co-glycolide) microspheres: how does the composition of the polymer matrices affect the time interval between the initial burst and the pulsatile release of drugs. Colloid Surf B 19:173–179
Manchanda S, PK Sahoo, DK Majumdar (2016a) Mucoadhesive chitosan-dextran sulphate nanoparticles of acetazolamide for ocular hypertension. Nanotechnol Rev 5:445–453
Manchanda S, PK Sahoo, DK Majumdar (2016b) Effect of formulation factors on transcorneal permeation of acetazolamide from aqueous drops: in vitro and in vivo study. Asian J Pharm 10:177–182
Mishra V, Jain NK(2014) Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm 461:380–390
Mohammadpourdounighi, N et al (2010) Preparation of chitosan nanoparticles containing NAJA naja oxiana snake venom. Nanomedicine 6:137–143
Palma SD et al (2009) An efficient ternary complex of acetazolamide with HP-β-CD and TEA for topical ocular administration. J Control Rel 138:24–31
Papadimitriou S et al (2008) Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohyd Polym 73: 44–54
Pawar, PK, DK Majumdar (2006) Effect of formulation factors on in vitro permeation of moxifloxacin from aqueous drops through excised goat, sheep, and buffalo corneas. AAPS Pharmsci 7:1–6
Ramin B et al (2016) Food hydrocolloids preparation and optimization of pickering emulsion stabilized by chitosan-tripolyphosphate nanoparticles for curcumin encapsulation. Food Hydrocolloid 52:369–377
Rathore MS, DK Majumdar (2006) Effect of formulation factors on in vitro transcorneal permeation of gatifloxacin from aqueous drops. AAPS Pharmsci 7:3–8
Rosu MC, I Bratu (2014) Promising psyllium-based composite containing TiO2 nanoparticles as aspirin-carrier matrix. Prog Nat Sci Mat Int 24:205–209
Varma VNSK et al (2014) Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm J 22:591–599
Acknowledgements
Authors are thankful to Prof. D.K. Majumdar (Apeejay Satya University) for his intellectual support and Prof. K. Sreenivas, Director, University Science Instrumentation Centre (USIC), University of Delhi (North campus), Delhi (India) for granting permission to use TEM and PXRD facilities. The authors (Satish Manchanda and Dr. Pravat Kumar Sahoo) report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manchanda, S., Sahoo, P.K. Fabrication and characterization of mucoadhesive topical nanoformulations of dorzolamide HCl for ocular hypertension. J. Pharm. Investig. 48, 323–332 (2018). https://doi.org/10.1007/s40005-017-0324-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-017-0324-x