Abstract
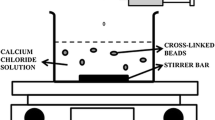
Regional absorption of several drugs necessitates the continuous monitoring of the movement of dosage form through gastro-intestinal tract. Sometimes it is important to deliver the drug at a particular physiological region of the gastrointestinal tract for better effects. Floating delivery system is a prominent approach to release the drug in the gastric fluid. The objective of present study was to formulate, evaluate and optimize cefdinir loaded intra-gastric floating beads of sodium alginate. Floating alginate beads were prepared by ionic gelation method according to Box–Behnken design with three factors varied at three levels. Uniform beads buoyant up to 24 h were formed with rough surface and porous internal structure. Characterization by Fourier transform infrared spectroscopy, differential scanning calorimetry, thermo-gravimetric analysis and powder x-ray diffractometry suggested an excellent compatibility of drug with excipients and formulation process. The effect of selected independent variables [amount of sodium alginate (X1), myristyl alcohol (X2) and cefdinir (X3) each at three levels] on the dependent variables [density (Y1), entrapment efficiency (Y2), time to release 20 % (Y3), cumulative percentage of cefdinir released at 12th hour (Y4) and dissolution efficiency (Y5)] were studied using regression equations and response surface plots. The predicted and adjusted r 2 values were in reasonable agreement and the models were significant with p < 0.05. Criteria were set for each responses and optimized formulation was prepared according to the software determined levels. The predicted and observed responses were in good agreement with low percent bias errors (<10 %), marking the validity of the developed model. Thus, cefdinir loaded, extended release, intra-gastric floating gel beads of calcium alginate were formulated and optimized.







Similar content being viewed by others
References
Ahsan MN, Verma PRP, Singh SK, Samridhi, Yashpal M (2014) Formulation of rosuvastatin-loaded self-nanoemulsifying drug delivery system using Box–Behnken design. Particul Sci Technol 32(1):46–60
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475
Box GEP, Wilson KB (1951) On the experimental attainment of optimum multifactorial conditions. R Stat Soc 13:1–12
Boza A, De la Cruz Y, Jordan G, Jauregui-Haza U, Aleman A, Caraballo I (2000) Statistical optimization of a sustained-release matrix tablet of lobenzarit disodium. Drug Dev Ind Pharm 26:1303–1307
Cabri W, Ghetti P, Alpegiani M et al (2006) Cefdinir: a comparative study of anhydrous vs. monohydrate form microstructure and tabletting behaviour. Eur J Pharm Biopharm 64:212–221
Cabri W, Ghetti P, Pozzi G, Alpegiani M (2007) Polymorphisms and patent, market, and legal battles: cefdinir case study. Org Process Res Dev 11:64–72
Chavanpatil M, Jain P, Chaudhari S, Shear R, Vavia PR (2005) Development of sustained release gastroretentive drug delivery system for ofloxacin: in vitro and in vivo evaluation. Int J Pharm 304:178–184
Cho HJ, Lee DW, Marasini N, Poudel BK, Kim JH, Ramasamy T, Yoo BK, Choi H-G, Yong CS, Kim JO (2013) Optimization of self-microemulsifying drug delivery system for telmisartan using Box–Behnken design and desirability function. J Pharm Pharmacol 65:1440–1450
El-Gibali I (2002) Development and in vitro evaluation of novel floating chitosan microcapsules for oral use: comparison with non-floating chitosan microspheres. Int J Pharm 249:7–21
Gannu R, Yamsani VV, Yamsani SK, Palem CR, Yamsani MR (2009) Optimization of hydrogels for transdermal delivery of lisinopril by Box–Behnken statistical design. AAPS PharmSciTech 10(2):505–514
Garg R, Gupta GD (2008) Progress in controlled gastroretentive delivery systems. Trop J Pharm Res 7(3):1055–1066
Gohel MC, Amin AF (1998) Formulation optimization of controlled release diclofenac sodium microspheres using factorial design. J Control Release 51:115–122
Guay DRP (2000a) Cefdinir: an expanded-spectrum oral cephalosporin. Ann Pharmacother 34(12):1469–1477
Guay DRP (2000b) Pharmacodynamics and pharmacokinetics of cefdinir, an oral extended spectrum cephalosporin. Pediatr Infect Dis J 19(12):S141–S146
Hoffman A, Stepensky D, Lavy E, Eyal S, Klausner E, Friedman M (2004) Pharmacokinetic and pharmacodynamic aspects of gastroretentive dosage forms. Int J Pharm 277:141–153
Iannuccelli V, Coppi G, Bernabei MT, Cameroni R (1998) Air compartment multiple-unit system for prolonged gastric residence, Part I: formulation study. Int J Pharm 174:47–54
Ma N, Xu L, Wang Q, Zhang X, Zhang W, Li Y, Jin L, Li S (2008) Development and evaluation of new sustained release floating microspheres. Int J Pharm 358:82–90
Mohit V, Harshal G, Neha D, Vilasrao K, Rajashree H (2010) Effect of preparation method on complexation of cefdinir with b-cyclodextrin. J Incl Phenom Macrocycl Chem 67:39–47
Murata Y, Sasaki N, Miyamoto E, Kawashima S (2000) Use of floating alginate gel beads for stomach-specfic drug delivery. Eur J Pharm Biopharm 50:221–226
Palamakula A, Nutan MTH, Khan MA (2004) Response surface methodology for optimization and characterization of limonene-based coenzyme Q10 self-nanoemulsified capsule dosage form. AAPS PharmSciTech 5(4):1–8
Praveen R, Singh SK, Verma PRP, George JK (2014) Sustained delivery of cefdinir to upper gastrointestinal tract using calcium alginate beads: a formulation by design. J Pharm Investig 44(6):455–463
Ragonese R, Macka M, Hughes J, Petocz P (2002) The use of the Box–Behnken experimental design in the optimization and robustness testing of a capillary electrophoresis method for the analysis of ethambutol hydrochloride in a pharmaceutical formulation. J Pharm Biomed Anal 27:995–1007
Saini R, Singh SK, Verma PRP (2014) Evaluation of carvedilol-loaded microsponges with nanometric pores using response surface methodology. J Exp Nanosci 9(8):831–850
Sanchez-Lafuente C, Furlanetto S, Fernandez-Arevalo M et al (2002) Didanosine extended-release matrix tablets: optimization of formulation variables using statistical experimental design. Int J Pharm 237:107–118
Sastry SV, Khan MA (1998) Aqueous based polymeric dispersion: plackett–Burman design for screening of formulation variables of atenolol gastrointestinal therapeutic system. Pharm Acta Helv 73:105–112
Singh SK, Dodge J, Durrani MJ, Khan MA (1995) Optimization and characterization of controlled release pellets coated with experimental latex: I. Anionic drug. Int J Pharm 125:243–255
Stops F, Fell JT, Collet JH, Martini LG (2008) Floating dosage forms to prolong gastro-retention- the characterization of calcium alginate beads. Int J Pharm 350:301–311
Tsuji A, Tamai I, Nakanishi M, Terasaki T, Hamano S (1993) Intestinal brush-border transport of the oral cephalosporin antibiotic, cefdinir, mediated by dipeptide and monocarboxylic acid transport systems in rabbits. J Pharm Pharmacol 45:996–998
Whitehead L, John H, Collett JH, Fell JT (2000) Amoxycillin release from a floating dosage form based on alginates. Int J Pharm 210:45–49
Acknowledgments
This article does not contain any studies with human and animal subjects performed by any of the authors. All authors (Radhakrishnan Praveen, Sandeep Kumar Singh, Priya Ranjan Prasad Verma and Jerome Karippamattom George) declare that they have no conflict of interest. The authors are grateful to Sance Laboratories Pvt. Ltd., Kerala, India for providing gift samples of CFD. The authors are also thankful to Birla Institute of Technology, Mesra, Ranchi, India and its central instrumentation facility for providing necessary facilities for the conduct of this work. One of the authors, Radhalrishnan Praveen, is thankful to UGC, New Delhi, India for financial assistance in the form of basic scientific research fellowship (Ref.: F.7-32/2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Praveen, R., Verma, P.R.P., Singh, S.K. et al. Cross linked alginate gel beads as floating drug delivery system for cefdinir: optimization using Box–Behnken design. Journal of Pharmaceutical Investigation 45, 187–199 (2015). https://doi.org/10.1007/s40005-014-0164-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0164-x