Abstract
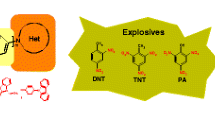
New fluorophores based on pyrimidines, bearing a pyrene electron-donating fragment, have been designed, synthesized and characterized. The fluorescence behavior of these fluorophores has been evaluated toward nitroaromatic compounds, including such well-known explosives, as 2,4-dinitroanisole, picric and styphnic acids, 1,3,5-triethoxy-2,4,6-trinitrobenzene, 2,4-dinitrotoluene, 2,4,6-trinitrotoluene, 2,4,6-triamino-1,3,5-trinitrobenzene and nitrobenzene. The detection limits for these fluorophores in relation to nitroaromatic compounds in acetonitrile solution proved to be in the range from 10−1 to 10−7 mol/L. The sensor prototypes for handheld sniffer «Nitroscan» (Plant «Promautomatika», Ekaterinburg, Russia) based on these fluorophores have been fabricated and used for applications, as illustrated by reusable, reversible and sensitive fast detection of nitrobenzene and 2,4,6-trinitrotoluene in vapor phase concentrations up to 6 and 5 ppb, respectively.
Graphical Abstract









Similar content being viewed by others
References
T.M. Figueira-Duarte, K. Müllen, Chem. Rev. 111, 7260 (2011)
R. Zhang, Y. Zhao, T. Zhang, L. Xu, Z. Ni, Dyes Pigments 130, 106 (2016). and references cited therein
Y. Hu, Y. Liu, G. Kim, E.J. Jun, K.M.K. Swamy, Y. Kim, S.-J. Kim, J. Yoon, Dyes Pigments 113, 372 (2015)
G. Xu, H. Wu, X. Liu, R. Feng, Z. Liu, Dyes Pigments 120, 322 (2015)
X. Zhou, J. Kim, Z. Liu, S. Jo, Y.L. Pak, K.M.K. Swamy, J. Yoon, Dyes Pigments 128, 256 (2016)
L. Dinga, Y. Fang, Chem. Soc. Rev. 39, 4258 (2010)
N. Leventis, I.A. Elder, D.R. Rolison, M.L. Anderson, C.I. Merzbacher, Chem. Mater. 11, 2837 (1999)
Y. Fujiwara, Y. Amao, Sens. Actuators B 89, 58 (2003)
Y.-X. Yuan, H.-S. Peng, J.-T. Ping, X.-H. Wang, F.-T. You, BioMed Res. Int. Article ID 245031, 6 pages (2015)
A. Ueno, I. Suzuki, T. Osa, Anal. Chem. 62, 2461 (1990)
J. Huang, Y. Wu, Y. Chen, Z. Zhu, X. Yang, C.J. Yang, K. Wang, W. Tan, Angew. Chem. Int. Ed. 50, 401 (2011)
B.A. Babgi, A. Alzahrani, J. Fluoresc. 26, 1415 (2016)
Y. Suzuki, T. Morozumi, H. Nakamura, M. Shimomura, T. Hayashita, R.A. Bartsh, J. Phys. Chem. B 102, 7910 (1998)
R.-H. Yang, W.-H. Chan, A.W.M. Lee, P.-F. Xia, H.-K. Zhang, K.A. Li, J. Am. Chem. Soc. 125, 2884 (2003)
S.C. Deshmukh, S. Rana, S.V. Shinde, B. Dhara, N. Ballav, P. Talukdar, ACS Omega 1, 371 (2016)
R.V. Taudte, A. Beavis, L. Wilson-Wilde, C. Roux, P. Doblea, L. Blanes, Lab Chip 13, 4164 (2013)
A. Senthamizhan, A. Celebioglu, S. Bayir, M. Gorur, E. Doganci, F. Yilmaz, T. Uyar, A.C.S. Appl, Mater. Interfaces 7, 21038 (2015)
I.S. Kovalev, O.S. Taniya, N.V. Slovesnova, G.A. Kim, S. Santra, G.V. Zyryanov, D.S. Kopchuk, A. Majee, V.N. Charushin, O.N. Chupakhin, Chem. Asian J. 11, 775 (2016)
G.V. Zyryanov, D.S. Kopchuk, I.S. Kovalev, E.V. Nosova, V.L. Rusinov, O.N. Chupakhin, Russ. Chem. Rev. 83, 783 (2014)
P. Beyazkilic, A. Yildirim, M. Bayindir, A.C.S. Appl, Mater. Interfaces 6, 4997 (2014)
S. Shanmugaraju, P.S. Mukherjee, Chem. Commun. 51, 16014 (2015)
J. Yinon, Counterterrorist detection techniques of explosives, 1st edn. (Elsevier B.V, Amsterdam, 2007), pp. 1–455
K.L. Diehl, E.V. Anslyn, Chem. Soc. Rev. 42, 8596 (2013)
Y. Salinas, R. Martínez-Máñez, M.D. Marcos, F. Sancenón, A.M. Costero, M. Parra, S. Gil, Chem. Soc. Rev. 41, 1261 (2012)
K.E. Brown, M.T. Greenfield, S.D. McGrane, D.S. Moore, Anal. Bioanal. Chem. 408, 35 (2016)
K.E. Brown, M.T. Greenfield, S.D. McGrane, D.S. Moore, Anal. Bioanal. Chem. 408, 49 (2016)
S. Giannoukos, B. Brkić, S. Taylor, A. Marshall, G.F. Verbeck, Chem. Rev. 116, 8146 (2016)
Website of the portable detector of nitro-explosives «Nitroscan»: http://nitroscan.pro
S.J. Toal, W.C. Trogler, J. Mater. Chem. 16, 2871 (2006)
W. Guan, W. Zhou, J. Lu, C. Lu, Chem. Soc. Rev. 44, 6981 (2015)
M.E. Germain, M.J. Knapp, Chem. Soc. Rev. 38, 2543 (2009)
M.S. Meaney, V.L. McGuffin, Anal. Bioanal. Chem. 391, 2557 (2008)
E.V. Verbitskiy, A.A. Baranova, K.I. Lugovik, M.Z. Shafikov, K.O. Khokhlov, E.M. Cheprakova, G.L. Rusinov, O.N. Chupakhin, V.N. Charushin, Anal. Bioanal. Chem. 408, 4093 (2016)
E.V. Verbitskiy, A.A. Baranova, K.I. Lugovik, K.O. Khokhlov, E.M. Cheprakova, G.L. Rusinov, O.N. Chupakhin, V.N. Charushin, ARKIVOC iii, 360 (2016)
E.V. Verbitskiy, E.B. Gorbunov, A.A. Baranova, K.I. Lugovik, K.O. Khokhlov, E.M. Cheprakova, G.A. Kim, G.L. Rusinov, O.N. Chupakhin, V.N. Charushin, Tetrahedron 72, 4954 (2016)
E.V. Verbitskiy, E.M. Cheprakova, A.A. Baranova, K.O. Khokhlov, K.I. Lugovik, G.L. Rusinov, O.N. Chupakhin, V.N. Charushin, Chem. Heterocycl. Compd. 52, 904 (2016)
E.V. Verbitskiy, A.A. Baranova, K.I. Lugovik, K.O. Khokhlov, E.M. Cheprakova, M.Z. Shafikov, G.L. Rusinov, O.N. Chupakhin, V.N. Charushin, Dyes Pigments 137, 360 (2017)
E.V. Verbitskiy, E.M. Cheprakova, P.A. Slepukhin, M.I. Kodess, M.A. Ezhikova, M.G. Pervova, G.L. Rusinov, O.N. Chupakhin, V.N. Charushin, Tetrahedron 68, 5445 (2012)
E.V. Verbitskiy, P.A. Slepukhin, M.S. Valova, E.M. Cheprakova, A.V. Schepochkin, G.L. Rusinov, V.N. Charushin, Eur. J. Org. Chem. 36, 8133 (2014)
A.M. Brouwer, Pure Appl. Chem. 83, 2213 (2011)
A.A. Baranova, K.O. Khokhlov, J. Phys: Conf. Ser. 552, 012034 (2014)
E.V. Verbitskiy, A.A. Baranova, Y.A. Yakovleva, R.D. Chuvashov, K.O. Khokhlov, E.M. Dinastiya, G.L. Rusinov, O.N. Chupakhin, V.N. Charushin, ARKIVOC v, 341 (2017)
B. Roy, A.K. Bar, B. Gole, P.S. Mukherjee, J. Org. Chem. 78, 1306 (2013)
Y.-J. Qu, J. Li, Inorg. Chem. Commun. 76, 77 (2017)
X. Tian, H. Peng, Y. Li, C. Yang, Z. Zhou, Y. Wang, Sens. Actuators B 243, 1002 (2017)
Acknowledgements
The authors are grateful to the Russian Foundation for Basic Research (Research Project No. 17-03-00011-A) for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Details of fluorescence quenching studies and copies of 1 H and 13 C NMR spectra of the products.
Rights and permissions
About this article
Cite this article
Verbitskiy, E.V., Baranova, A.A., Lugovik, K.I. et al. Linear and V-shaped push–pull systems on a base of pyrimidine scaffold with a pyrene-donative fragment for detection of nitroaromatic compounds. J IRAN CHEM SOC 15, 787–797 (2018). https://doi.org/10.1007/s13738-017-1278-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1278-7