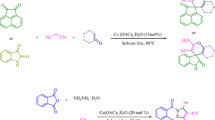
Abstract
This article aimed to present two facile and environmental friendly routes for the rapid assembly of biologically active compounds including pyrazol core using aspirin as a novel and green catalyst. The synthesis of bis(pyrazol-5-ol) derivatives was developed via one-pot, pseudo-five-component condensation, and the target dihydropyrano[2,3-c]pyrazoles and spiropyranopyrazoles were prepared by one-pot, four-component reaction. These reactions can be performed in tandem from readily available starting materials. The main merits of the present methods are operational simplicity, no need for column chromatography, inexpensive materials, avoidance of harmless and corrosive acid catalysts, short reaction times, good yields of the products, and utilization of aspirin as a non-toxic, cheap, commercially available, and efficient catalyst.
Graphical abstract




Similar content being viewed by others
References
S. Karamthulla, S. Pal, M.N. Khan, L.H. Choudhury, RSC Adv. 4, 37889–37899 (2014)
P.T. Anastas, T.C. Williamson (eds.), Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes (Oxford University Press, Oxford, 1998)
G.M. Ziarani, S. Faramarzi, N. Lashgari, A. Badiei, J. Iran. Chem. Soc. 11, 701–709 (2014)
P.T. Anastas, M.M. Kirchhoff, Acc. Chem. Res. 35, 686–694 (2002)
E. Ruijter, R. Scheffelaar, R.V.A. Orru, Angew. Chem. Int. Ed. 50, 6234–6246 (2011)
J.L. Tucker, Org. Process Res. Dev. 10, 315–319 (2006)
O.A. Attanasi, D. Spinelli, Soc. Chim. Italiana, Roma. 4, 105–137 (2000)
M.M.F. Ismail, Y.A. Ammar, H.S.A. EI-Zahaby, S.I. Eisa, S.E. Barakat, Arch. Pharm. Chem. Life Sci. 340, 476–479 (2007)
S.P. Prajapati, D.P. Patel, P.S. Patel, J. Chem. Pharm. Res. 4, 2652–2655 (2012)
Y. Liu, G. He, C. Kai, Y. Li, H. Zhu, J. Heterocycl. Chem. 19, 1370–1375 (2012)
G. Mariappan, B.P. Saha, L. Sutharson, A. Singh, S. Garg, L. Pandey, D. Kumar, Saudi Pharm. J. 19, 115–122 (2011)
C.E. Rosiere, M.I. Grossman, Science 131, 651 (1951)
M.G. LaPorte, Z. Wang, R. Colombo, A. Garzan, V.A. Peshkov, M. Liang, P.A. Johnston, M.E. Schurdak, M. Sen, D.P. Camarco, Y. Hua, Bioorg. Med. Chem. Lett. 26, 3581–3588 (2016)
J.L. Wang, D. Liu, Z.J. Zheng, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S., Z. Huang. Proc. Natl. Acad. Sci. 97, 7124–7129 (2009)
B.P. Bandgar, H.V. Chavan, L.K. Adsul, V.N. Thakare, S.N. Shringare, R. Shaikh, R.N. Gacche, Bioorg. Med. Chem. Lett. 23, 912–916 (2013)
K.M. Kasiotis, E.N. Tzanetou, S.A. Haroutounian, Front. Chem. 2, 1–7 (2014)
M.J. Genin, C. Biles, B.J. Keiser, S.M. Poppe, S.M. Swaney, W.G. Tarpley, Y. Yagi, D.L. Romero, J. Med. Chem. 43, 1034–1040 (2000)
F. Abrigach, R. Touzani, Med. Chem. (Los Angeles) 6, 292–298 (2016)
K. Sujatha, G. Shanthi, N.P. Selvam, S. Manoharan, P.T. Perumal, M. Rajendran, Bioorg. Med. Chem. Lett. 19, 4501–4503 (2009)
H. Kashtoh, M.T. Muhammad, J.J. Khan, S. Rasheed, A. Khan, S. Perveen, K. Javaid, K.M. Khan, M.I. Choudhary, Bioorg. Chem. 65, 61–72 (2016)
A.V. Stachulski, N.G. Berry, A.C.L. Low, S.L. Moores, E. Row, D.C. Warhurst, I.S. Adagu, J.F. Rossignol, J. Med. Chem. 49, 1450–1454 (2006)
E. Soleimani, S. Ghorbani, M. Taran, A. Sarvary, C. R. Chim. 15, 955–961 (2012)
J. Safaei-Ghomi, B. Khojastehbakht-Koopaei, H. Shahbazi-Alavi, RSC Adv. 4, 46106–46113 (2014)
W. Wang, S.X. Wang, X.Y. Qin, J.T. Li, Synth. Commun. 35, 1263–1269 (2005)
N.G. Khaligh, S.B.A. Hamid, S.J. Titinchi, Chin. Chem. Lett. 27, 104–108 (2016)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, V. Khakyzadeh, S. Azizian, J. Mol. Catal. A-Chem. 418–419, 54–67 (2016)
D. Azarifar, Y. Abbasi, Synth. Commun. 46, 745–758 (2016)
Y.A. Tayade, S.A. Padvi, Y.B. Wagh, D.S. Dalal, Tetrahedron Lett. 56, 2441–2447 (2015)
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 7559–7579 (2016)
C.F. Zhou, J.J. Li, W.K. Su, Chin. Chem. Lett. 27, 1686–1690 (2016)
A.K. Imene, F. Amina, L. Oumeima, B. Raouf, B. Boudjemaa, D. Abdelmadjid, Lett. Org. Chem. 13, 85–91 (2016)
H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523–2525 (2011)
A. Siddekha, A. Nizam, M.A. Pasha, Spectrochim. Acta A. 81, 431–440 (2011)
A.R. Hajipour, M. Karimzadeh, H. Tavallaei, J. Iran. Chem. Soc. 12, 987–991 (2015)
K. Ablajan, W. Liju, Y. Kelimu, F. Jun, Mol. Divers. 17, 693–700 (2013)
D.W.C. MacMillan, Nature 455, 304–308 (2008)
C.M. Ulrich, J. Bigler, J.D. Potter, Nat. Rev. Cancer 6, 130–140 (2006)
C. Cena, M.L. Lolli, L. Lazzarato, E. Guaita, G. Morini, G. Coruzzi, S.P. McElroy, I.L. Megson, R. Fruttero, A. Gasco, J. Med. Chem. 46, 747–754 (2003)
A. Undas, K.E. Brummel-Ziedins, K.G. Mann, Blood 109, 2285–2592 (2007)
X. Shi, M. Ding, Z. Dong, F. Chen, J. Ye, S. Wan, S.S. Leonard, V. Castronova, V. Vallyathan, Mol. Cell. Biochem. 199, 93–102 (1999)
T. Roberts, F. Shokraneh, S. Nur, Cochrane Libr (2016). doi:10.1002/14651858.CD012116
M. Fatahpour, F. Noori Sadeh, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, Res. Appl. Chem. 6, 1569–1572 (2016)
M. Kangani, N. Hazeri, M.T. Mghsoodlou, K. Khandan-Barani, M. Kheyrollahi, F. Nezhadshahrokhabadi, J. Iran. Chem. Soc. 12, 47–50 (2015)
M. Fatahpour, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, Iran. J. Sci. Technol. Trans. A Sci. (2016). doi:10.1007/s40995-016-0064-1
F. Mohamadpour, M.T. Maghsoodlou, R. Heydari, M. Lashkari, J. Iran. Chem. Soc. 13, 1549–1560 (2016)
M.T. Maghsoodlou, M. Karima, M. Lashkari, B. Adrom, J. Aboonajmi, J. Iran. Chem. Soc. 14, 329–335 (2017)
J. Xu-dong, D. Hai-feng, L. Ying-jie, C. Jun-gang, L. Da-peng, W. Mao-cheng, Chem. Res. Chin. Univ. 28, 999–1002 (2012)
M. Babaie, H. Sheibani, Arab. J. Chem. 11, 159–162 (2011)
Y. Zou, H. Wub, Y. Hua, H. Liu, X. Zhao, H. Ji, D. Shi, Ultrason. Sonochem. 18, 708–712 (2011)
B. Maleki, H. Eshghi, M. Barghamadi, N. Nasiri, A. Khojastehnezhad, S. Sedigh Ashrafi, O. Pourshiani, Res. Chem. Intermed. 42, 3071–3093 (2016)
D.R. Palleros, Experimental Organic Chemistry (Wiley, Hoboken, 2000), p. 494
Y. Zou, Y. Hu, H. Liu, D. Shi, ACS comb. Sci. 14, 38–43 (2011)
S. Paul, K. Pradhan, S. Ghosh, S.K. De, A.R. Das, Tetrahedron 70, 6088–6099 (2014)
M.N. Elinson, A.I. Ilovaisky, V.M. Merkulova, P.A. Belyakov, F. Barba, B. Batanero, Tetrahedron 68, 5833–5837 (2012)
Acknowledgements
We gratefully appreciate the financial support from the Research Council of University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatahpour, M., Noori Sadeh, F., Hazeri, N. et al. Aspirin: an efficient catalyst for synthesis of bis (pyrazol-5-ols), dihydropyrano[2,3-c]pyrazoles and spiropyranopyrazoles in an environmentally benign manner. J IRAN CHEM SOC 14, 1945–1956 (2017). https://doi.org/10.1007/s13738-017-1133-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1133-x