Abstract
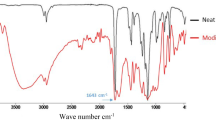
Polar species of natural rubber (NR) core were produced from PMPS-g-NR using ozonolysis. Poly(3-(trimethoxysilyl) propyl methacrylate) (PMPS) coated on NR particles (PMPS-g-NR) was successfully prepared by surface grafting polymerization using a redox couple initiator. PMPS-g-NR was treated by ozone at different feeding rates (50, 75, 100, and 125 mg/h) and treatment times (15, 30, 60, and 120 min) leading to an increase in polar function. Fourier transform infrared spectrometry (FTIR) showed that unsaturated bond (840 cm−1) was consumed by ozone resulting in higher intensities of carbonyl and carboxyl groups. The highest percent gel content of ozonized PMPS-g-NR was found at 100 mg/h at each ozone treatment time. After preparation as a polymer electrolyte, the electrical properties were significantly improved. Ozonized PMPS-g-NR showed that the highest ionic conductivity of ozonized PMPS-g-NR was 1.96 mS cm−1 using an ozone feeding rate of 75 mg/h for 60 min while PMPS-g-NR was 0.41 mS cm−1. The solar cell conversion efficiency (ƞ) of ozonized PMPS-g-NR at 75 mg/h for 60 min was 1.51% having a higher value than PMPS-g-NR (0.35%). For thermal stress stability, normalized ƞ value of ozonized PMPS-g-NR remained at 6.25% after 26 days while normalized ƞ of PMPS-g-NR was 27.27% at 22 days and then reduced to an approaching zero value.










Similar content being viewed by others
References
Grätzel M (2003) Dye-sensitized solar cells. J Photochem Photobiol C Photochem Rev 4:145–153
Wei D (2010) Dye sensitized solar cells. Int J Mol Sci 11:1103–1113
Maabong K, Muiva CM, Monowe P, Sathiaraj TS, Hopkins M, Nguyen L, Malungwa K, Thobega M (2015) Natural pigments as photosensitizers for dye-sensitized solar cells with TiO2 thin films. Int J Renew Energy Res 5:54–60
Jawad M, Al-Ajaj E, Suhail M, Majid S (2014) Efficiency enhancement of photovoltaic performance of quasisolid state dye sensitized solar cell with TPAI and KI binary iodide salt mixture. Adv Phys Theor Appl 34:51–59
Bandara T, Svensson T, Dissanayake M, Furlani M, Jayasundara W, Fernando PSL, Albinsson I, Mellander BE (2013) Conductivity behaviour in novel quasi-solid-state electrolyte based on polyacrylonitrile and tetrahexylammonium iodide intended for dye sensitized solar cells. J Natl Sci Found Sri Lanka 41:175–184
Bandara TMWJ, Fernando HDNS, Furlani M, Albinsson I, Dissanayake MAKL, Ratnasekera JL, Mellander B-E (2017) Dependence of solar cell performance on the nature of alkaline counterion in gel polymer electrolytes containing binary iodides. J Solid State Electrochem 21:1571–1578
Caruso F (2001) Nanoengineering of particle surfaces. Adv Mater 13:11–22
Sakhare MS, Rajput H (2017) Polymer grafting and applications in pharmaceutical drug delivery systems—a brief review. Asian J Pharm Clin Res 10:59–63
Zhou T, Zhu Y, Li X, Liu X, Yeung KWK, Wu S, Wang X, Cui Z, Yang X, Chu PK (2016) Surface functionalization of biomaterials by radical polymerization. Prog Mater Sci 83:191–235
Bai H, Huang Z, Yang W (2009) Visible light-induced living surface grafting polymerization for the potential biological applications. J Polym Sci Polym Chem 47:6852–6862
Aramesh M, Cervenka J (2015) Surface modification of porous anodic alumina for medical and biological applications. In: Seifalian A, Mel AD, Kalaskar DM (eds) Nanomedicine, 1st edn. One Central Press (OCP), Manchester
Kangwansupamonkon W, Gilbert RG, Kiatkamjornwong S (2005) Modification of natural rubber by grafting with hydrophilic vinyl monomers. Macromol Chem Phys 206:2450–2460
Oliveira PC, Guimarães A, Cavaillé J-Y, Chazeau L, Gilbert RG, Santos AM (2005) Poly(dimethylaminoethyl methacrylate) grafted natural rubber from seeded emulsion polymerization. Polymer 46:1105–1111
Lamb DJ, Anstey JF, Fellows CM, Monteiro MJ, Gilbert RG (2001) Modification of natural and artificial polymer colloids by “topology-controlled” emulsion polymerization. Biomacromolecules 2:518–525
Sim LN, Arof AK (2017) Elastomers and their potential as matrices in polymer electrolytes. In: Cankaya N (ed) Elastomers. IntechOpen, London
Ali AMM, Subban RHY, Bahron H, Yahya MZA, Kamisan AS (2013) Investigation on modified natural rubber gel polymer electrolytes for lithium polymer battery. J Power Sourc 244:636–640
Kamisan AS, Kudin TIT, Ali AMM, Yahya MZA (2011) Electrical and physical studies on 49% methyl-grafted natural rubber-based composite polymer gel electrolytes. Electrochim Acta 57:207–211
Kalkornsurapranee E, Vennemann N, Kummerlöwe C, Nakason C (2012) Novel thermoplastic natural rubber based on thermoplastic polyurethane blends: influence of modified natural rubbers on properties of the blends. Iran Polym J 21:689–700
Thongnuanchan B, Rattanapan S, Persalea K, Thitithammawong A, Pichaiyut S, Nakason C (2017) Improving properties of natural rubber/polyamide 12 blends through grafting of diacetone acrylamide functional group. Polym Adv Technol 28:1148–1155
Tahir M, Heinrich G, Mahmood N, Boldt R, Wießner S, Stöckelhuber KW (2018) Blending in situ polyurethane-urea with different kinds of rubber: performance and compatibility aspects. Materials 11:2175
Arayapranee W, Rempel GL (2013) Effects of polarity on the filler–rubber interaction and properties of silica filled grafted natural rubber composites. J Polym 2013:1–9
Zamri S, Abdul Latif F, Izzati Husna Mohd Azuan S, Ibrahim R, Hadip F, Kamaluddin N, Ali AMM (2018) Filler and polymer interactions in polymethyl methacrylate/50% epoxidized natural rubber/silicon dioxide nanocomposites. Malays J Anal Sci 22:586–593
Mensah B, Kang SI, Wang W, Nah C (2018) Effect of graphene on polar and nonpolar rubber matrices. Mech Adv Mater Modern Proc 4:1
Syahidah Hussin N, Harun F, Han Chan C (2017) Thermal properties and conductivity of thermally treated epoxidized natural rubber-based solid polymer electrolytes. Macromol Symp 376:1700049
Klinklai W, Kawahara S, Mizumo T, Yoshizawa M, Sakadapipanich JT, Isono Y, Ohno H (2003) Depolymerization and ionic conductivity of enzymatically deproteinized natural rubber having epoxy group. Eur Polym J 39:1707–1712
Lin Q, Lu YB, Ren WT, Zhang Y (2015) The grafting reaction of epoxidized natural rubber with carboxyl ionic liquids and the ionic conductivity of solid electrolyte composites. RSC Adv 5:90031–90040
Fainleib A, Pires RV, Lucas EF, Soares BG (2013) Degradation of non-vulcanized natural rubber—renewable resource for fine chemicals used in polymer synthesis. Polimeros 23:441–450
Joseph J, Son KM, Vittal R, Lee W, Kim K-J (2006) Quasi-solid-state dye-sensitized solar cells with siloxane poly(ethylene glycol) hybrid gel electrolyte. Semicond Sci Technol 21:697–701
Zhao J, Jo S-G, Kim D-W (2014) Photovoltaic performance of dye-sensitized solar cells assembled with electrospun polyacrylonitrile/silica-based fibrous composite membranes. Electrochim Acta 142:261–267
Yang Y, Hu H, Zhou C-H, Xu S, Sebo B, Zhao X-Z (2011) Novel agarose polymer electrolyte for quasi-solid state dye-sensitized solar cell. J Power Source 196:2410–2415
Bourgeat-Lami E, Tissot I, Lefebvre F (2002) Synthesis and characterization of SiOH-functionalized polymer latexes using methacryloxy propyl trimethoxysilane in emulsion polymerization. Macromolecules 35:6185–6191
Cataldo F, Ursini O, Angelini G (2010) Surface oxidation of rubber crumb with ozone. Polym Degrad Stabil 95:803–810
Fisher TJ, Dussault PH (2017) Alkene ozonolysis. Tetrahedron 73:4233–4258
Kurtén T, Bonn B, Vehkamäki H, Kulmala M (2007) Computational study of the reaction between biogenic stabilized Criegee intermediates and sulfuric acid. J Phys Chem A 111:3394–3401
Huang D, Chen ZM, Zhao Y, Liang H (2013) Newly observed peroxides and the water effect on the formation and removal of hydroxyalkyl hydroperoxides in the ozonolysis of isoprene. Atmos Chem Phys 13:5671–5683
Nor HM, Ebdon JR (2000) Ozonolysis of natural rubber in chloroform solution. Part 1. A study by GPC and FTIR spectroscopy. Polymer 41:2359–2365
Miwa S, Kikuchi T, Ohtake Y, Tanaka K (2011) Surface degradation of poly(ethylene-co-propylene-co-5-ethylidene-2-norbornene) terpolymer by ozone in water. Polym Degrad Stabil 96:1503–1507
Rodrigues FHA, Santos EF, Feitosa JPA, Ricardo N, Heatley F (2004) Ozonation of unstretched natural rubber film from Hevea brasiliensis studied by ozone consumption and 13C NMR. Polym Int 53:733–739
Rakovsky S, Anachkov M, Zaikov G (2009) Fields of ozone applications. Chem Chem Technol 3:139–161
Zhang D, Zhang R (2002) Mechanism of OH formation from ozonolysis of isoprene: a quantum-chemical study. J Am Chem Soc 124:2692–2703
Kolsaker P (1978) Formation of cis and trans ozonides from Cinnamic esters. Acta Chem Scand B 32:557–560
Aziz SB (2013) Li+ ion conduction mechanism in poly(ε-caprolactone)-based polymer electrolyte. Iran Polym J 22:877–883
Promdum Y, Klinpituksa P, Ruamcharoen J (2009) Grafting copolymerization of natural rubber with 2-hydroxyethyl methacrylate for plywood adhesion improvement. SJST 31:453–457
Lee W-F, Chen C-Y (2015) Graft copolymerization of 3-(trimethoxysilyl) propyl methacrylate onto styrene–butadiene–styrene triblock copolymer. J Elastom Plast 47:103–116
Mohapatra S, Nando GB (2013) Chemical modification of natural rubber in the latex stage by grafting cardanol, a waste from the Cashew Industry and a renewable resource. Ind Eng Chem Res 52:5951–5957
Tan KS, Yusof A (2003) Some studies on the effect of solvents in ENR 60 gel content measurements. J Rubber Res 6:189–194
Wongthong P, Nakason C, Pan Q, Rempel GL, Kiatkamjornwong S (2013) Modification of deproteinized natural rubber via grafting polymerization with maleic anhydride. Eur Polym J 49:4035–4046
Lee W-F, Chen C-Y (2015) Graft copolymerization of 3-(trimethoxysilyl) propyl methacrylate onto styrene–butadiene–styrene triblock copolymer. J Elast Plast 47:103–116
Tham WL, Chow WS, Ishak ZAM (2010) The effect of 3-(trimethoxysilyl) propyl methacrylate on the mechanical, thermal, and morphological properties of poly(methyl methacrylate)/hydroxyapatite composites. J Appl Polym Sci 118:218–228
Smith BC (1999) Infrared spectral interpretation: a systematic approach. CRC Press LLC, Boca Raton
Sassi Z, Bureau JC, Bakkali A (2002) Spectroscopic study of TMOS–TMSM–MMA gels: previously identification of the networks inside the hybrid material. Vib Spectrosc 28:299–318
Simionescu B, Olaru M, Aflori M, Cotofana C (2010) Silsesquioxane-based hybrid nanocomposite with self-assembling properties for porous limestones conservation. High Perform Polym 22:42–55
Sasitaran M, Manroshan S, Lim CS, Krishna Veni BN, Ong SK, Gunasunderi R (2017) Preparation and characterisation of crosslinked natural rubber (SMR CV 60) and epoxidised natural rubber (ENR-50) blends. ASEAN J Sci Technol Develop 34:106–118
Carretero-González J, Ezquerra TA, Amnuaypornsri S, Toki S, Verdejo R, Sanz A, Sakdapipanich J, Hsiao BS, López-Manchado MA (2010) Molecular dynamics of natural rubber as revealed by dielectric spectroscopy: the role of natural cross-linking. Soft Matter 6:3636–3642
Ramli R, Jaapar J, Singh M, Md. Yatim AH (2014) Physical properties and fatigue lifecycles of natural rubber latex gloves. Adv Environ Biol 8:2714–2722
Giglio ED, Ditaranto N, Sabbatini L (2014) Polymer surface chemistry: characterization by XPS. In: Sabbatini L (ed) Polymer surface characterization. Walter de Gruyter GmbH, Berlin
Costa MJ, Marques AM, Pastrana LM, Teixeira JA, Sillankorva SM, Cerqueira MA (2018) Physicochemical properties of alginate-based films: effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocolloids 81:442–448
Peinado C, Corrales T, Catalina F, Pedrón S, Santa Quiteria VR, Parellada MD, Barrio JA, Olmos D, González-Benito J (2010) Effects of ozone in surface modification and thermal stability of SEBS block copolymers. Polym Degrad Stabil 95:975–986
Nagaraj P, Sasidharan A, David V, Sambandam A (2017) Effect of cross-linking on the performances of starch-based biopolymer as gel electrolyte for dye-sensitized solar cell applications. Polymers 9:667
Acknowledgements
This work was supported by Center of Excellence on Petrochemical and Materials Technology, Bangkok, Thailand; and the Petroleum and Petrochemical College, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silakul, P., Magaraphan, R. A polymer electrolyte by ozonolysis of poly(3-(trimethoxysilyl) propyl methacrylate) grafted on natural rubber latex in colloid state and its application. Iran Polym J 28, 455–470 (2019). https://doi.org/10.1007/s13726-019-00714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-019-00714-6