Abstract
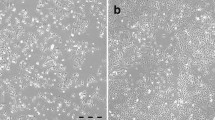
Malignant peritoneal mesothelioma (MPeM) is a rare and aggressive form of malignant mesothelioma. Sufficient biological tools for studying the functional characteristics of this cancer have not been developed. Therefore, in this study, a novel human cancer cell line, KOG-1, was established from ascites fluids isolated from a 39-year-old Japanese woman with pemetrexed-resistant MPeM. Cells were dendritic or linear immediately after thawing, showed a jigsaw puzzle-like and spindle arrangement during growth, and formed monolayers without contact inhibition in two-dimensional (2D) culture. The population doubling time was 13.7 h. Karyotypic and molecular genetic analyses showed that chromosome numbers ranged from 62 to 142, with a peak of 73 with complicated copy number alterations. No germline BAP1 pathogenic variant was detected. Cells expressed various tumor markers of mesothelioma, such as calretinin, podoplanin, and Wilms tumor 1 (WT-1). Drug sensitivity and resistance testing with a set of 36 drugs using 2D and three-dimensional (3D) culture models demonstrated that KOG-1 cells showed high and low sensitivity to pemetrexed under 2D and 3D culture conditions, respectively, whereas control ovarian cancer cell lines showed low sensitivity to pemetrexed under both culture conditions. This newly established cell line will be a valuable biological resource to expand the feasibility of functional studies as well as drug testing for potential therapeutic purposes in MPeM.







Similar content being viewed by others
References
Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, Borczuk AC, Butnor K, Cagle PT, Chirieac LR, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647–67.
Husain AN, Colby TV, Ordonez NG, Allen TC, Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med. 2018;142:89–108.
Rodriguez D, Cheung MC, Housri N, Koniaris LG. Malignant abdominal mesothelioma: defining the role of surgery. J Surg Oncol. 2009;99:51–7.
Mensi C, Mendola M, Dallari B, Sokooti M, Tabibi R, Riboldi L, Consonni D. Differences between peritoneal and pleural mesothelioma in Lombardy, Italy. Cancer Epidemiol. 2017;51:68–73.
Baratti D, Kusamura S, Deraco M. Diffuse malignant peritoneal mesothelioma: systematic review of clinical management and biological research. J Surg Oncol. 2011;103:822–31.
Bianchi C, Brollo A, Ramani L, Bianchi T, Giarelli L. Asbestos exposure in malignant mesothelioma of the pleura: a survey of 557 cases. Ind Health. 2001;39:161–7.
Facchetti G, Petrella F, Spaggiari L, Rimoldi I. Malignant pleural mesothelioma: state of the art and advanced cell therapy. Eur J Med Chem. 2017;142:266–70.
Garcia-Fadrique A, Mehta A, Mohamed F, Dayal S, Cecil T, Moran BJ. Clinical presentation, diagnosis, classification and management of peritoneal mesothelioma: a review. J Gastrointest Oncol. 2017;8:915–24.
Hirasawa A, Imoto I, Naruto T, Akahane T, Yamagami W, Nomura H, Masuda K, Susumu N, Tsuda H, Aoki D. Prevalence of pathogenic germline variants detected by multigene sequencing in unselected Japanese patients with ovarian cancer. Oncotarget. 2017;8:112258–67.
Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402.
Wang J, Wang W, Li R, Li Y, Tian G, Goodman L, Fan W, Zhang J, Li J, Zhang J, et al. The diploid genome sequence of an Asian individual. Nature. 2008;456:60–5.
Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, He W, Chen YJ, Makhijani V, Roth GT, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–6.
Yoshii Y, Furukawa T, Waki A, Okuyama H, Inoue M, Itoh M, Zhang MR, Wakizaka H, Sogawa C, Kiyono Y, et al. High-throughput screening with nanoimprinting 3D culture for efficient drug development by mimicking the tumor environment. Biomaterials. 2015;51:278–89.
Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–6.
Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 2004;10:1032–42.
Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW, Whang-Peng J, Rogan AM, Green WR, Ozols RF. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983;43:5379–89.
Tange T, Hasegawa Y, Oka T, Sunaga S, Higashihara M, Matsuo K, Miyazaki H, Shimosaka A, Okano A, Todokoro K. Establishment and characterization of a new human mesothelioma cell line (T-85) from malignant peritoneal mesothelioma with remarkable thrombocytosis. Pathol Int. 1995;45:791–800.
Oumi N, Itamochi H, Komatsu H, Oishi T, Shimada M, Sato S, Chikumi J, Sato S, Nonaka M, Kudoh A, Harada T. Establishment and mutation analysis of a novel malignant peritoneal mesothelioma cell line, TU-MM-1, using whole genome sequencing. Hum Cell. 2016;29:46–51.
Li Q, Verschraegen CF, Mendoza J, Hassan R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1 (dsFv) PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004;24:1327–35.
Attanoos RL, Churg A, Galateau-Salle F, Gibbs AR, Roggli VL. Malignant mesothelioma and its non-asbestos causes. Arch Pathol Lab Med. 2018;142:753–60.
https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/tokusyu/chuuhisyu15/dl/chuuhisyu.pdf. Accessed 27 May 2019.
Gneral rules for clinical and pathological record of mesothelial tumors November 2018 (The 1st Edition) Japan asbestos mesothelioma study group, Japan mesothelioma interest group, the Japan lung cancer society. Kanehara & CO., Ltd., Tokyo, JAPAN, 2018.11
Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16:5517–27.
Nabavi N, Wei J, Lin D, Collins CC, Gout PW, Wang Y. Pre-clinical models for malignant mesothelioma research: from chemical-induced to patient-derived cancer xenografts. Front Genet. 2018;9:232.
Morotti M, Valenzano Menada M, Venturini PL, Mammoliti S, Ferrero S. Pemetrexed disodium in ovarian cancer treatment. Expert Opin Investig Drugs. 2012;21:437–49.
Roche M, Parisi L, Li L, Knehans A, Phaeton R, Kesterson JP. The role of pemetrexed in recurrent epithelial ovarian cancer: a scoping review. Oncol Rev. 2018;12:346.
Janne PA, Wozniak AJ, Belani CP, Keohan ML, Ross HJ, Polikoff JA, Mintzer DM, Ye Z, Monberg MJ, Obasaju CK. Pemetrexed expanded access program i: pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac Oncol. 2006;1:506–12.
van den Bogaert DP, Pouw EM, van Wijhe G, Vernhout RM, Surmont VF, Hoogsteden HC, van Klaveren RJ. Pemetrexed maintenance therapy in patients with malignant pleural mesothelioma. J Thorac Oncol. 2006;1:25–30.
Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Do P, Bylicki O, Monnet I, Corre R, Audigier-Valette C, Locatelli-Sanchez M, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–53.
Hassan R, Alley E, Kindler H, Antonia S, Jahan T, Honarmand S, Nair N, Whiting CC, Enstrom A, Lemmens E, et al: Live-Attenuated, Listeria monocytogenes Expressing Mesothelin (CRS-207) with Chemotherapy for Treatment of Malignant Pleural Mesothelioma. Clin Cancer Res 2019.
Papa S, Popat S, Shah R, Prevost AT, Lal R, McLennan B, Cane P, Lang-Lazdunski L, Viney Z, Dunn JT, et al. Phase 2 study of sorafenib in malignant mesothelioma previously treated with platinum-containing chemotherapy. J Thorac Oncol. 2013;8:783–7.
Dubey S, Janne PA, Krug L, Pang H, Wang X, Heinze R, Watt C, Crawford J, Kratzke R, Vokes E, Kindler HL. A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307. J Thorac Oncol. 2010;5:1655–61.
Ugurluer G, Chang K, Gamez ME, Arnett AL, Jayakrishnan R, Miller RC, Sio TT. Genome-based mutational analysis by next generation sequencing in patients with malignant pleural and peritoneal mesothelioma. Anticancer Res. 2016;36:2331–8.
Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, Gnad F, Nguyen TT, Jaiswal BS, Chirieac LR, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–16.
Betti M, Aspesi A, Biasi A, Casalone E, Ferrante D, Ogliara P, Gironi LC, Giorgione R, Farinelli P, Grosso F, et al. CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett. 2016;378:120–30.
Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5.
Ohar JA, Cheung M, Talarchek J, Howard SE, Howard TD, Hesdorffer M, Peng H, Rauscher FJ, Testa JR. Germline BAP1 mutational landscape of asbestos-exposed malignant mesothelioma patients with family history of cancer. Cancer Res. 2016;76:206–15.
Haugh AM, Njauw CN, Bubley JA, Verzi AE, Zhang B, Kudalkar E, VandenBoom T, Walton K, Swick BL, Kumar R, et al. Genotypic and phenotypic features of BAP1 cancer syndrome: a report of 8 new families and review of cases in the literature. JAMA Dermatol. 2017;153:999–1006.
Husain AN, Colby TV, Ordonez NG, Allen TC, Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med. 2018;142:89–108.
Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–49.
Acknowledgements
We thank the patient and the supporting medical staff for making this study possible. We are also grateful to Ms. Tomomi Noda, Ms. Atsuko Fukushima, and Ms. Shihomi Yamaguchi for administrative assistance and biobank data management, and Mr. Ippei Sakamoto and Mr. Shigeki Tanishima for NGS data management.
Funding
This work was supported by the JSPS Bilateral program and the KAKENHI (Grant Nos. 18K09298, 19K09833).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We have no conflicts of interest to declare for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akahane, T., Hirasawa, A., Imoto, I. et al. Establishment and characterization of a new malignant peritoneal mesothelioma cell line, KOG-1, from the ascitic fluid of a patient with pemetrexed chemotherapy resistance. Human Cell 33, 272–282 (2020). https://doi.org/10.1007/s13577-019-00286-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00286-w