Abstract
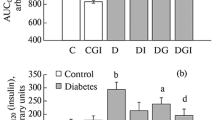
Previous studies have shown that Bacillus-produced surfactin (SFN) can be used for oral delivery of insulin (INS). To improve the bioavailability of INS, we determined the effects of SFN on intranasal delivery of INS in diabetic mice. Combinations of SFN and INS at different doses were used for intranasal administration of diabetic mice. The plasma levels of glucose and INS were determined at various time intervals after intranasal administration, and then, the hypoglycemic effects and relative bioavailability of INS were calculated. Glucose tolerance test was performed to determine the effects of intranasal delivery of INS plus SFN on the control of glucose levels. Diabetic mice were also intranasally administered with the INS and SFN combo for 7 days to determine the short-term stability of this formulation for controlling blood glucose levels. A combination of 20 IU/kg INS and 1.6 mg/kg SFN achieved the best hypoglycemic effects for intranasal administration, with a maximal hypoglycemic rate of 29.59% and a maximal blood INS concentration of 45.47 μIU/ml 2 h after administration. As a result, a relatively increased bioavailability of 8.55% was achieved. Glucose tolerance test showed that intranasal delivery of INS plus SFN could effectively control the blood glucose levels after the influx of glucose. Furthermore, intranasal INS plus SFN could be used for controlling blood glucose daily for a short term. Histological evaluation showed no changes in the morphology of the nasal mucosa after exposure to SFN plus INS. SFN is potentially useful for intranasal delivery of INS to control blood glucose levels.






Similar content being viewed by others
References
Duan X, Mao S. New strategies to improve the intranasal absorption of insulin. Drug Discov Today. 2010;15:416–27.
Benedict C, Brede S, Schiöth HB, Lehnert H, Schultes B, Born J, et al. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes. 2011;60:114–8.
Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes. 2015;64:766–74.
De la Mont SM. Intranasal insulin therapy for cognitive impairment and neurodegeneration: current state of the art. Expert Opin Drug Deliv. 2013;10:1699–709.
Patton JS, Platz RM. Routes of delivery: case studies. Adv Drug Dely Rev. 1992;8:179.
Wang Y, Zhang X, Cheng C, Li C. Mucoadhesive and enzymatic inhibitory nanoparticles for transnasal insulin delivery. Nanomedicine. 2014;9:451–64.
Carrillo C, Teruel JA, Aranda FJ, Ortiz A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta. 2003;1611:91–7.
Maget-Dana R, Ptak M. Interactions of surfactin with membrane models. Biophys J. 1995;68(5):1937–43.
Zou A, Liu J, Garamus VM, Zheng K, Willumeit R, Mu B. Interaction between the natural lipopeptide (Glu1, Asp5) Surfactin-C15 and hemoglobin in aqueous solution. Biomacromolecules. 2010;11:593–9.
Cameotra S, Makkar R. Recent applications of biosurfactants as biological and immunological molecules. Curr Opin Microbiol. 2004;7(3):262–6.
Hwang YH, Kim MS, Song IB, Park BK, Lim JH, Park SC, et al. Subacute (28 day) toxicity of surfactin C, a lipopeptide produced by Bacillus, in rats. J Health Sci. 2009;55:351–5.
Zhang L, Gao Z, Zhao X, Qi G. A natural lipopeptide of surfactin for oral delivery of insulin. Drug Deliv. 2016;23:2084–93.
Qi G, Zhu F, Du P, Yang X, Qiu D, Yu Z, et al. Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides. 2010;31:1978–86.
Makhlof A, Tozuka Y, Takeuchi H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur J Pharm Sci. 2011;42:445–51.
Rekha MR, Sharma CP. Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J Control Release. 2009;135:144–51.
Sajeesh S, Bouchemal K, Marsaud V, Vauthier C, Sharma CP. Cyclodextrin complexes insulin encapsulated hydrogel microparticles: an oral delivery system for insulin. J Control Release. 2010;147:377–84.
Sonaje K, Lin YH, Juang JH, Wey SP, Chen CT, Sung HW. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials. 2009;30:2329–39.
Carino GP, Jacob JS, Mathiowitz E. Nanosphere-based oral insulin delivery. J Control Release. 2000;65:261–9.
Damgé C, Maincent P, Ubrich N. Oral delivery of insulin associated with polymeric nanoparticles in diabetic rats. J Control Release. 2007;117:163–70.
Furtado S, Abramson D, Burrill R, Olivier G, Gourd C, Bubbers E, et al. Oral delivery of insulin-loaded poly (fumaric-co-sebacic) anhydride microspheres. Int J Pharm. 2008;347:149–55.
Kim SK, Lee S, Jin S, Moon HT, Jeon OC, Lee DT, et al. Diabetes correction in pancreatectomized canines by the orally absorbable insulin-deoxycholate complex. Mol Pharm. 2010;7:708–17.
Daniel C, Weigmann B, Bronson R, Von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mime tope. J Exp med. 2011;208:1501–10.
Sintov AC, Levy HV, Botner S. Systemic delivery of insulin via the nasal route using a new microemulsion system: in vitro and in vivo studies. J Control Release. 2010;148:168–76.
Rima BS, Manhar D, David MM, Viral NS. Insulin delivery methods: past, present and future. Int J Pharm Investig. 2016;6:1–9.
Heinemann L, Jacques Y. Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Technol. 2009;3:568–84.
Zhang Y, Jiang XG, Yao J. Nasal absorption enhancement of insulin by sodium deoxycholate in combination with cyclodextrins. Acta Pharmacol Sin. 2001;22:1051–6.
Gordon GS, Moses AC, Silver RD, Flier JS, Carey MC. Nasal absorption of insulin: enhancement by hydrophobic bile salts. Proc Natl Acad Sci U S A. 1985;82:7419–23.
Shao Z, Li Y, Chermak T, Mitra A. Cyclodextrins as mucosal absorption promoters of insulin. II. Effects of β-cyclodextrin derivatives on α-chymotryptic degradation and enteral absorption of insulin in rats. Pharm Res. 1994;11:1174–9.
Leary AC, Dowling M, Cussen K, O’Brien J, Stote RM. Pharmacokinetics and pharmacodynamics of intranasal insulin spray (NasulinTM) administered to healthy male volunteers: influence of the nasal cycle. J Diabetes Sci Technol. 2008;2:1054–60.
Gao Z, Zhao X, Lee S, Li J, Liao H, Zhou X, et al. WH1fungin a surfactin cyclic lipopeptide is a novel oral immunoadjuvant. Vaccine. 2013;31:2796–803.
Kim J, Park S, Kang HM, Ahn CW, Kwon HC, Song JH, et al. Human insulin secreted from insulinogenic xenograft restores normoglycemia in type 1 diabetic mice without immunosuppression. Cell Transplant. 2012;21:2131–47.
Author information
Authors and Affiliations
Contributions
G Qi designed the study. Q Yu, S Dong, and X Xing executed the experimental work. D Yang analyzed the data. X Zhao contributed reagents and materials. Q Yu and G Qi wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This study was funded by the Fundamental Research Funds for the Central Universities (Program No. 2016BC016).
Conflict of interest
The authors declared that there are no conflicts of interest.
Human and animal rights
All applicable University Ethics Committee’s guidelines for the care and use of animals were followed.
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yu, Q., Dong, S., Yang, D. et al. Bacillus-produced surfactin for intranasal delivery of insulin in diabetic mice. Int J Diabetes Dev Ctries 38, 321–329 (2018). https://doi.org/10.1007/s13410-017-0564-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-017-0564-3