Abstract
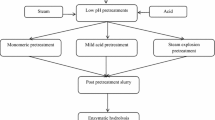
The development of micro-assays suitable for automation systems represents a helpful alternative by which many enzymes and factors can be studied simultaneously for the conversion of lignocellulose. The purpose of the present study was to evaluate a micro-assay method, based on hand-sheets, to reproducibly aliquot-pretreated lignocellulosic biomass at milligramme scale with minimal impact on the digestibility of the material. To favour representative sampling and handling for enzymatic conversion at milligramme scale, the solid fractions from steam-pretreated sweet sorghum bagasse (SSB) and triticale straw (TS) were subjected to homogenisation steps in sequential disintegrator and liquidiser at different severities prior to hand-sheet making (TAPPI). In the case of SSB, the disintegrator treatment (37,500 rpm) was sufficient to obtain the required fibre lengths, while the combined treatment with liquidiser (6250 rpm) and disintegrator (31,250 rpm) was needed for TS. The selected steps were able to improve homogenisation and fibre size distribution in the hand-sheets, attaining the required fibre lengths (less than 8 mm), with minimal influence on glucan conversion when using a conventional enzyme cocktail. The combined treatment and hand-sheet making had a more pronounced effect on the conversion of the xylan, with reduced xylose yields compared to small scale. The carbohydrate conversion in micro-assay was susceptible to more efficient enzyme combinations depending on the structural properties of the pretreated materials, presence and concentration of inhibitors as well as the enzyme dosage used. Nevertheless, the micro-assay method could distinguish between the different enzyme combinations, identifying those resulting in higher sugar yields from different lignocellulose materials pretreated under relatively mild conditions.





Similar content being viewed by others
References
Guo H, Chang Y, Lee D (2018) Enzymatic saccharification of lignocellulosic biorefinery: research focuses. Bioresour Technol 252:198–215. https://doi.org/10.1016/j.biortech.2017.12.062
Benjamin Y, García-Aparicio M, Görgens JF (2014) Impact of cultivar selection and process optimization on ethanol yield from different varieties of sugarcane. Biotechnol Biofuels 7(60):1–17. https://doi.org/10.1186/1754-6834-7-60
Benjamin Y, Cheng H, Görgens JF (2013) Evaluation of bagasse from different varieties of sugarcane by dilute acid pretreatment and enzymatic hydrolysis. Ind Crop Prod 51:7–18. https://doi.org/10.1016/j.indcrop.2013.08.067
Benjamin Y, Cheng H, Görgens JF (2014) Optimization of dilute sulfuric acid pretreatment to maximize combined sugar yield from sugarcane bagasse for ethanol production. Appl Biochem Biotechnol 172:610–630. https://doi.org/10.1007/s12010-013-0545-z
Viikari L, Vehmaanpera J, Koivula A (2012) Lignocellulosic ethanol: from science to industry. Biomass Bioenergy 46:13–24. https://doi.org/10.1016/j.biombioe.2012.05.008
Decker SR, Harman-Ware AE, Happs RM, Wolfrum EJ, Tuskan GA, Kainer D, Oguntimein G, Rodríguez M, Weighill D, Jones PC, Jacobson D (2018) High throughput screening technologies in biomass characterization. Front Energy Res 6:120. https://doi.org/10.3389/fenrg.2018.00120
Selig MJ, Tucker MP, Sykes RW, Reichel KL, Brunecky R, Himmel ME, Davis MF, Decker SR (2010) Lignocellulose recalcitrance screening by integrated high throughput hydrothermal pretreatment and enzymatic saccharification. Ind Biotechnol 6:104–111
Decker SR, Sykes RW, Turner GB, Lupoi JS, Doeppke C, Tucker MP, Schuster LA, Mazza K, Himmel ME, Davis MF, Gjersing E (2015) High-throughput screening of recalcitrance variations in lignocellulosic biomass: total lignin, lignin monomers, and enzymatic sugar release. J Vis Exp (103):e53163. https://doi.org/10.3791/53163
Gao X, Kumar R, Demartini JD, Li H, Wyman CE (2013) Application of high throughput pretreatment and co-hydrolysis system to thermochemical pretreatment. Part 1 : dilute acid. Biotechnol Bioeng 110:754–762. https://doi.org/10.1002/bit.24751
Gomez LD, Whitehead C, Barakate A, Halpin C, Mcqueen-mason SJ (2010) Automated saccharification assay for determination of digestibility in plant materials. Biotechnol Biofuels 3:23. https://doi.org/10.1186/1754-6834-3-23
Li H, Gao X, Demartini JD, Kumar R, Wyman CE (2013) Application of high throughput pretreatment and co-hydrolysis system to thermochemical pretreatment. Part 2: dilute alkali. Biotechnol Bioeng 110:2894–2901. https://doi.org/10.1002/bit.24951
Navarro D, Couturier M, Ghizzi G, Berrin J, Rouau X, Asther M, Bignon C (2010) Automated assay for screening the enzymatic release of reducing sugars from micronized biomass. Microb Cell Factories 9(58):1–12. https://doi.org/10.1186/1475-2859-9-58
Song L, Laguerre S, Dumon C, Bozonnet S, Donohue MJO (2010) A high-throughput screening system for the evaluation of biomass-hydrolyzing glycoside hydrolases. Bioresour Technol 101:8237–8243. https://doi.org/10.1016/j.biortech.2010.05.097
Banerjee G, Car S, Scott-Craig JS, Borrusch MS, Walton JD (2010) Rapid optimization of enzyme mixtures for deconstruction of diverse pretreatment/biomass feedstock combinations. Biotechnol Biofuels 3:1–15. https://doi.org/10.1186/1754-6834-3-22
Malgas S, Chandra R, Van Dyk JS, Saddler JN, Pletschke BI (2017) Formulation of an optimized synergistic enzyme cocktail, HoloMix, for effective degradation of various pre-treated hardwoods. Bioresour Technol 245:52–65. https://doi.org/10.1016/j.biortech.2017.08.186
Hernández-Beltrán JU, Cervantes-Quintero K, Hernández-Escoto H (2018) A quick and effective method for evaluating substrate-enzyme systems in the enzymatic hydrolysis of lignocellulosic biomass. Biomass Convers Bior 8:437–446. https://doi.org/10.1007/s13399-017-0297-z
Badhan A, Wang Y, Gruninger R, Patton D, Powlowski J, Tsang A, McAllister T (2014) Formulation of enzyme blends to maximize the hydrolysis of alkaline peroxide pretreated alfalfa hay and barley straw by rumen enzymes and commercial cellulases. BMC Biotechnol 14:1–14. https://doi.org/10.1186/1472-6750-14-31
Berlin A, Maximenko V, Bura R, Kang K, Gilkes N, Saddler J (2005) A rapid microassay to evaluate enzymatic hydrolysis of lignocellulosic substrates. Biotechnol Bioeng 93:880–886. https://doi.org/10.1002/bit
Alvira P, Negro MJ, Sáez F, Ballesteros M (2010) Application of a microassay method to study enzymatic hydrolysis of pretreated wheat straw. J Chem Technol Biotechnol 85:1291–1297. https://doi.org/10.1002/jctb.2430
Agudelo R, García-Aparicio M, Görgens J (2016) Steam explosion pretreatment of triticale (× Triticosecale Wittmack) straw for sugar production. New Biotechnol 33:153–163. https://doi.org/10.1016/j.nbt.2015.10.001
Mcintosh PA (2013). Selection of preferred sweet sorghum cultivars and their pretreatment optimization for bioethanol production. MSc Thesis, Stellenbosch University
Pengilly C, García-Aparicio MP, Diedericks D, Brienzo M, Görgens JF (2015) Enzymatic hydrolysis of steam-pretreated sweet sorghum bagasse by combinations of cellulase and endo-xylanase. Fuel 154:352–360. https://doi.org/10.1016/j.fuel.2015.03.072
Pengilly C, García-Aparicio MP, Diedericks D, Görgens JF (2016) Optimization of enzymatic hydrolysis of steam pretreated triticale straw. Bioenergy Res 9:851–863. https://doi.org/10.1007/s12155-016-9741-3
Guo H, Wang X, Lee D (2018b) Proteomic researches for lignocellulose-degrading enzymes: a mini-review. Bioresour Technol 265:532–541. https://doi.org/10.1016/j.biortech.2018.05.101
Jones DR, Thomas D, Alger N, Ghavidel A, Inglis GD, Abbott DW (2018) SACCHARIS: an automated pipeline to streamline discovery of carbohydrate active enzyme activities within polyspecific families and de novo sequence datasets. Biotechnol Biofuels 11(27):1–15. https://doi.org/10.1186/s13068-018-1027-x
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008). Determination of sugars, byproducts, and degradation products in liquid fraction process samples. NREL/TP-510-42623
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012). Determination of structural carbohydrates and lignin in biomass. NREL/TP-510-42618 analytical procedure. Laboratory Analytical Procedure (LAP). https://doi.org/NREL/TP-510-42618
TAPPI (2002). Forming hand-sheets for physical tests of pulp. Test Method TAPPI/ANSI T 205 sp-18, TAPPI
Chambers JM, Cleveland WS, Kleiner B, Tukey PA (1983) Graphical methods for data analysis. Wadsworth, Belmont
Cantarella M, Cantarella L, Gallifuoco A, Spera A (2004) Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol Prog 20:200–206. https://doi.org/10.1021/bp0257978
García-Aparicio MP, Ballesteros I, González A, Oliva JM, Ballesteros M, Negro MJ (2006) Effect of inhibitors released during steam-explosion pretreatment of barley straw on enzymatic hydrolysis. Appl Biochem Biotechnol 129:278–288. https://doi.org/10.1385/abab:129:1:278
Qing Q, Yang B, Wyman CE (2010) Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour Technol 101:9624–9630. https://doi.org/10.1016/j.biortech.2010.06.137
Zhai R, Hu J, Saddler JN (2018) The inhibition of hemicellulosic sugars on cellulose hydrolysis are highly dependent on the cellulase productive binding, processivity and substrate surface charges. Bioresour Technol 258:79–87. https://doi.org/10.1016/j.biortech.2017.12.006
Chundawat SPS, Balan V, Dale BE (2008) High-throughput microplate technique for enzymatic hydrolysis of lignocellulosic biomass. 99:1281–1294. https://doi.org/10.1002/bit.21805
Luo X, Zhu JY (2011) Effects of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses. Enzym Microb Technol 48:92–99. https://doi.org/10.1016/j.enzmictec.2010.09.014
Moreira Barbosa B, Luiz Colodotte J, Coelho dos Santos Muguet M, Juste Gomes V, Chaves De Oliveira R, (2016). Effects of xylan in Eucalyptus pulp production. CERNE. https://doi.org/10.1590/01047760201622022102
Hu J, Arantes V, Pribowo A, Gourlay K, Saddler JN (2014) Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ Sci 7:2308–2315. https://doi.org/10.1039/c4ee00891j
Ju X, Grego C, Zhang X (2013) Specific effects of fiber size and fiber swelling on biomass substrate surface area and enzymatic digestibility. Bioresour Technol 144:232–239. https://doi.org/10.1016/j.biortech.2013.06.100
Del Rio LF, Chandra RP, Saddler JN (2012) Fibre size does not appear to influence the ease of enzymatic hydrolysis of organosolv-pretreated softwoods. Bioresour Technol 107:235–242. https://doi.org/10.1016/j.biortech.2011.12.057
Brienzo M, Fikizolo S, Benjamin Y, Tyhoda L, Görgens J (2017) Influence of pretreatment severity on structural changes, lignin content and enzymatic hydrolysis of sugarcane bagasse samples. Renew Energy 104:271–280. https://doi.org/10.1016/j.renene.2016.12.037
Herbaut M, Zoghlami A, Paës G (2018) Dynamical assessment of fluorescent probes mobility in poplar cell walls reveals nanopores govern saccharification. Biotechnol Biofuels 11:271–213. https://doi.org/10.1186/s13068-018-1267-9
Gandla M, Martín C, Jönsson L (2018) Analytical enzymatic saccharification of lignocellulosic biomass for conversion to biofuels and bio-based chemicals. Energies 11:2936. https://doi.org/10.3390/en11112936
Acknowledgments
The authors thank Genencor and Novozymes for kindly donating the enzyme preparations. The authors thank Dr. Phumla Vena and Mr. Henry J. Solomon for their assistance with the hand-sheet preparation.
Funding
The Senior Chair of Energy Research (CoER) and National Research Foundation (NRF) provided financial support.
Author information
Authors and Affiliations
Contributions
All experiments were planned by CP in collaboration with MGA and JS and executed by CP. The results were analysed by CP with the help of MGA and JS. CP and MGA wrote the first draft of the manuscript. All co-authors gave feedback and contributed to draft the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pengilly, C., García-Aparicio, M., Swart, J.P.J. et al. Micro-assay method for enzymatic saccharification of industrially relevant lignocellulose substrates. Biomass Conv. Bioref. 12, 299–311 (2022). https://doi.org/10.1007/s13399-020-00700-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00700-6