Abstract
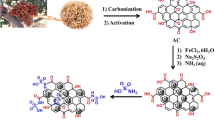
Extraction of pectin (Pec) from banana (Musa paradisiaca L.) peels, the agricultural waste of banana fruit treated in acidic condition, and its fabrication into pectin–activated carbon (Pec–AC) composite by impregnation method were investigated. This Pec–AC composite was applied as an adsorbent to simultaneously remove multi-lanthanides, namely yttrium (Y), lanthanum (La), cerium (Ce), neodymium (Nd), and samarium (Sm), which were released from an acidic silica sand aqueous solution. Various fractions of Pec in Pec–AC composite and the effects of different parameters on the adsorption capacity of the multi-lanthanides were also evaluated. At the optimum condition, the adsorption capacity of the lanthanides was 84.4, 77.7, 67.5, 57.5, and 53.7% for Y, Nd, Sm, Ce, and La, with respect to their content in the silica sand. The Qmax value was estimated to be 21.80, 27.78, 18.22, 21.09, and 24.91 mg g−1 for La3+, Y3+, Nd3+, Sm3+, and Ce3+, respectively. Fourier-transform infrared analysis suggests that the lanthanides are bound to the electronegative functional groups on the active sites of the Pec–AC composite. The simultaneous adsorption of the lanthanides on Pec–AC composite is governed by the pseudo-second-order kinetics, and the adsorption process of the lanthanides can be described by Temkin isotherm model. Overall results suggest in this study that the Pec–AC composite is an efficient potential adsorbent to simultaneously absorb multi-lanthanides from leachate of silica sand as the primary step of isolation of lanthanides.





Similar content being viewed by others
References
Jowitt, S.M.; Medlin, C.C.; Cas, R.A.F.: The rare earth element (REE) mineralisation potential of highly fractionated rhyolites: a potential low-grade, bulk tonnage source of critical metals. Ore Geol. Rev. 86, 548–562 (2017)
Iclodean, C.; Varga, B.; Burnete, N.; Cimerdean, D.; Jurchiş, B.: Comparison of different battery types for electric vehicles. IOP Conf. Ser. Mater. Sci. Eng. 252, 012058 (2017)
Lin, S.L.; Huang, K.L.; Wang, I.C.; Chou, I.C.; Kuo, Y.M.; Hung, C.H.; Lin, C.: Characterization of spent nickel–metal hydride batteries and a preliminary economic evaluation of the recovery processes. J. Air Waste Manag. Assoc. 66, 296–306 (2016)
Binnemans, K.; Jones, P.T.; Blanpain, B.; Gerven, T.V.; Yang, Y.-X.; Walton, A.; Buchert, M.: Recycling of rare earths: a critical review. J. Clean. Prod. 51, 1–22 (2013)
Farmer, T.J.; Clark, J.H.; Hunt, A.J.: Elemental sustainability and the importance of scarce element recovery. In: Hunt, A.J. (ed.) Element Recovery and Sustainability, pp. 1–28. RSC Publishing, Cambridge (2013)
Burakova, I.V.; Burakov, A.E.; Tkachev, A.G.; Troshkina, I.D.; Veselova, O.A.; Babkin, A.V.; Aung, W.M.; Ali, I.: Kinetics of the adsorption of scandium and cerium ions in sulfuric acid solutions on a nanomodified activated carbon. J. Mol. Liq. 253, 277–283 (2018)
Tanda, B.C.; Oraby, E.A.; Eksteen, J.J.: Recovery of copper from alkaline glycine leach solution using solvent extraction. Sep. Purif. Technol. 187, 389–396 (2017)
Reddy, K.R.; Nakata, K.; Ochiai, T.; Murakami, T.; Tryk, D.A.; Fujishima, A.: Nanofibrous TiO2–core/conjugated polymer–sheath composites: synthesis, structural properties and photocatalytic activity. J. Nanosci. Nanotechnol. 10, 7951–7957 (2010)
Reddy, K.R.; Hassan, M.; Gomes, V.G.: Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A Gen. 489, 1–16 (2015)
Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M.: Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 210, 850–866 (2019)
Morshed, M.N.; Bouazizi, N.; Behary, N.; Guan, J.; Nierstrasz, V.: Stabilization of zero valent iron (Fe0) on plasma/dendrimer functionalized polyester fabrics for Fenton-like removal of hazardous water pollutants. Chem. Eng. J. 374, 658–673 (2019)
Zheng, M.; Han, Y.; Xu, C.; Zhang, Z.; Han, H.: Selective adsorption and bioavailability relevance of the cyclic organics in anaerobic pretreated coal pyrolysis wastewater by lignite activated coke. Sci. Total Environ. 653, 64–73 (2019)
Awual, M.R.; Kobayashi, T.; Shiwaku, H.; Miyazaki, Y.; Motokawa, R.; Suzuki, S.; Okamoto, Y.; Yaita, T.: Evaluation of lanthanide sorption and their coordination mechanism by EXAFS measurement using novel hybrid adsorbent. Chem. Eng. J. 225, 558–566 (2013)
Kim, J.; Benjamin, M.M.: Modeling a novel ion exchange process for arsenic and nitrate removal. Water Res. 38, 2053–2062 (2004)
Koekkoek, A.J.J.; Kim, W.; Degirmenci, V.; Xin, H.; Ryoo, R.; Hensen, E.J.M.: Catalytic performance of sheet-like Fe/ZSM-5 zeolites for the selective oxidation of benzene with nitrous oxide. J. Catal. 299, 81–89 (2013)
Wu, L.; Degirmenci, V.; Magusin, P.C.M.M.; Szyja, B.M.; Hensen, E.J.M.: Dual template synthesis of highly mesoporous SSZ-13 zeolite with improved stability in the methanol-to-olefins reaction. Chem. Commun. 48, 9492–9494 (2012)
Hillen, L.; Degirmenci, V.: Synthesis methods for the production of hierarchically mesoporous and microporous zeolites. Rev. Adv. Sci. Eng. 4, 147–162 (2015)
Degirmenci, V.; Hensen, E.J.M.: Development of a heterogeneous catalyst for lignocellulosic biomass conversion: glucose dehydration by metal chlorides in a silica-supported ionic liquid layer. Environ. Prog. Sustain. Energy 33, 657–662 (2014)
Koekkoek, A.J.J.; Degirmenci, V.; Hensen, E.J.M.: Dry gel conversion of organosilane templated mesoporous silica: from amorphous to crystalline catalysts for benzene oxidation. J. Mater. Chem. 21, 9279–9289 (2011)
Wilson, L.D.; Mahmud, S.T.: The adsorption properties of surface-modified mesoporous silica materials with β-cylodextrin. Int. J. Technol. 4, 533–545 (2015)
Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Walton, R.I.; Degirmenci, V.: Exceptionally efficient and recyclable heterogeneous metal–organic framework catalyst for glucose isomerization in water. ChemCatChem 10, 706–709 (2018)
Zhang, Y.; Degirmenci, V.; Li, C.; Hensen, E.J.M.: Phosphotungstic acid encapsulated in metal-organic framework as catalyst for carbohydrate dehydration to 5-hydroxymethylfurfural. Chemsuschem 4, 59–64 (2011)
Iannicelli-Zubiani, E.M.; Stampino, P.G.; Cristiani, C.; Dotelli, G.: Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 341, 75–82 (2018)
Reddy, K.R.; Sin, B.C.; Ryu, K.S.; Noh, J.; Lee, Y.: In situ self-organization of carbon black-polyaniline composites from nanospheres to nanorods: synthesis, morphology, structure and electrical conductivity. Synth. Met. 159, 1934–1939 (2009)
Reddy, K.R.; Reddy, C.V.; Nadagouda, M.N.; Shetti, N.P.; Jaesool, S.; Aminabhavi, T.M.: Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: synthesis methods, properties and photocatalytic applications. J. Environ. Manag. 238, 25–40 (2019)
Khan, M.U.; Reddy, K.R.; Snguanwongchai, T.; Haque, E.; Gomes, V.G.: Polymer brush synthesis on surface modified carbon nanotubes via in situ emulsion polymerization. Colloid Polym. Sci. 294, 1599–1610 (2016)
Kumar, S.; Bukkitgar, S.D.; Singh, S.; Singh, P.V.; Reddy, K.R.; Shetti, N.P.; Reddy, C.V.; Sadhu, V.; Naveen, S.: Electrochemical sensors and biosensors based on graphene functionalized with metal oxide nanostructures for healthcare applications. Chem. Select. 4, 5322–5337 (2019)
Choi, Y.S.; Yeo, C.S.; Kim, S.J.; Lee, J.Y.; Kim, Y.; Cho, K.R.; Ju, S.; Hong, B.H.; Park, S.Y.: Multifunctional reduced graphene oxide-CVD graphene core–shell fibers. Nanoscale 11, 12637–12642 (2019)
Cakici, M.; Reddy, K.R.; Alonso-Marroquin, F.: Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chem. Eng. J. 309, 151–158 (2017)
Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Bagihalli, G.B.; Reddy, K.R.; Ravindranadh, K.; Reddy, C.V.: A novel biosensor based on graphene oxide-nanoclay hybrid electrode for the detection of Theophylline for healthcare applications. Microchem. J. 149, 103985 (2019)
Shetti, N.P.; Nayak, D.S.; Malode, S.J.; Kakarla, R.R.; Shukla, S.S.; Aminabhavi, T.M.: Sensors based on ruthenium-doped TiO2 nanoparticles loaded into multi-walled carbon nanotubes for the detection of flufenamic acid and mefenamic acid. Anal. Chim. Acta 1051, 58–72 (2019)
Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M.: Activated carbon modifications to enhance its water treatment applications: an overview. J. Hazard. Mater. 187, 1–23 (2011)
Zhang, Y.; Pan, K.; Zhong, Q.: Characteristics of activated carbon and carbon nanotubes as adsorbents to remove annatto (norbixin) in cheese whey. J. Agric. Food Chem. 61, 9230–9240 (2013)
Ren, X.; Chen, C.; Nagatsu, M.; Wang, X.: Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem. Eng. J. 170, 395–410 (2011)
Rao, G.P.; Lu, C.; Su, F.: Sorption of divalent metal ions from aqueous solution by carbon nanotubes: a review. Sep. Purif. Technol. 58, 224–231 (2007)
Ren, X.; Li, J.; Tan, X.; Wang, X.: Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 42, 5266–5274 (2013)
Ji, L.; Chen, W.; Duan, L.; Zhu, D.: Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 43, 2322–2327 (2009)
Czech, B.; Oleszczuk, P.: Sorption of diclofenac and naproxen onto MWCNT in model wastewater treated by H2O2 and/or UV. Chemosphere 149, 272–278 (2016)
Ma, C.Y.; Huang, S.C.; Chou, P.H.; Den, W.; Hou, C.H.: Application of a multiwalled carbon nanotube-chitosan composite as an electrode in the electrosorption process for water purification. Chemosphere 146, 113–120 (2016)
Esfahani, M.R.; Tyler, J.L.; Stretz, H.A.; Wells, M.J.M.: Effects of a dual nanofiller, nano-TiO2 and MWCNT for polysulfone-based nanocomposite membranes for water purification. Desalination 372, 47–56 (2015)
Yang, S.; Hu, J.; Chen, C.; Shao, D.; Wang, X.: Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ. Sci. Technol. 45, 3621–3627 (2011)
Pyrzynska, K.: Sorption of Cd(II) onto carbon-based materials: a comparative study. Microchim. Acta 169, 7–13 (2010)
Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J.: Activated carbon, carbon nanotubes and graphene: materials and composites for advanced water purification. J. Carbon Res. 3, 18 (2017)
Zahid, I.; Hussain, S.; Malghani, N.; Naeem, Z.; Amin, M.; Mushtaq, F.; Anwer, A.: Municipal wastewater treatment using rice husk and kikar. In: International Research Symposium on Engineering Advancements 2016, Sri Lanka, 2016, pp. 56–59
Liu, D.; Zhang, W.; Lin, H.; Li, Y.; Lu, H.; Wang, Y.: A green technology for the preparation of high capacitance rice husk-based activated carbon. J. Clean. Prod. 112, 1190–1198 (2016)
Kazemi, F.; Younesi, H.; Ghoreyshi, A.A.; Bahramifar, N.; Heidari, A.: Thiol-incorporated activated carbon derived from fir wood sawdust as an efficient adsorbent for the removal of mercury ion: batch and fixed-bed column studies. Process Saf. Environ. Protect. 100, 22–35 (2016)
Tao, H.C.; Zhang, H.R.; Li, J.B.; Ding, W.Y.: Biomass based activated carbon obtained from sludge and sugarcane bagasse for removing lead ion from wastewater. Bioresour. Technol. 192, 611–617 (2015)
Gafri, H.F.S.; Mohamed Zuki, F.; Aroua, M.K.; Hashim, N.A.: Mechanism of bacterial adhesion on ultrafiltration membrane modified by natural antimicrobial polymers (chitosan) and combination with activated carbon (PAC). Rev. Chem. Eng. 35, 421–443 (2019)
Belo, C.R.; Cansado, I.P.; Mourão, P.A.M.: Synthetic polymers blend used in the production of high activated carbon for pesticides removals from liquid phase. Environ. Technol. (UK) 38, 285–296 (2017)
Hokkanen, S.; Bhatnagar, A.; Sillanpaa, M.: A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 91, 156–173 (2016)
Wong, W.W.; Abbas, F.M.A.; Liong, M.T.; Azhar, M.E.: Modification of durian rind pectin for improved biosorbent ability. Int. Food Res. J. 15, 363–365 (2008)
Carpenter, A.W.; de Lannoy, C.F.; Wiesner, M.R.: Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 49, 5277–5287 (2015)
de Azevedo, A.C.N.; Vaz, M.G.; Gomes, R.F.; Pereira, A.G.B.; Fajardo, A.R.; Rodrigues, F.H.A.: Starch/rice husk ash based superabsorbent composite: high methylene blue removal efficiency. Iran. Polym. J. 26, 93–105 (2017)
Barakat, M.A.: New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 4, 361–377 (2011)
Malik, A.H.; Iyer, P.K.: Conjugated polyelectrolyte based sensitive detection and removal of antibiotics tetracycline from water. ACS Appl. Mater. Interface. 9, 4433–4439 (2017)
Luo, X.G.; Zeng, J.; Liu, S.L.; Zhang, L.N.: An effective and recyclable adsorbent for the removal of heavy metal ions from aqueous system: magnetic chitosan/cellulose microspheres. Bioresour. Technol. 194, 403–406 (2015)
Opanasopit, P.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U.: Development and characterization of pectinate micro/nanoparticles for gene delivery. AAPS PharmSciTech 9, 67–74 (2008)
Kusrini, E.; Wicaksono, W.; Gunawan, C.; Daud, N.Z.A.; Usman, A.: Kinetics, mechanism, and thermodynamics of lanthanum adsorption on pectin extracted from durian rind. J. Environ. Chem. Eng. 6, 6580–6588 (2018)
Mahesh, M.; Arivizhivendhan, K.V.; Maharaja, P.; Boopathy, R.; Hamsavathani, V.; Sekaran, G.: Production, purification and immobilization of pectinase from Aspergillus ibericus onto functionalized nanoporous activated carbon (FNAC) and its application on treatment of pectin containing wastewater. J. Mol. Catal. B Enzym. 133, 43–54 (2016)
Farahnaky, A.; Sharifi, S.; Imani, B.; Dorodmand, M.M.; Majzoobi, M.: Physicochemical and mechanical properties of pectin–carbon nanotubes films produced by chemical bonding. Food Packag. Shelf Life 16, 8–14 (2018)
Wu, F.-C.; Tseng, R.-L.; Juang, R.-S.: Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 150, 366–373 (2009)
Weber, W.J.; Morris, J.C.: Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 89, 31–60 (1963)
Boyd, G.E.; Adamson, A.W.; Myers Jr., L.S.: The exchange adsorption of ions from aqueous solutions by organic zeolites; II kinetics. J. Am. Chem. Soc. 69, 2836–2848 (1947)
Zaidi, N.A.H.M.; Lim, L.B.L.; Priyantha, N.; Usman, A.: Artocarpus odoratissimus leaves as an eco-friendly adsorbent for the removal of toxic rhodamine B dye in aqueous solution: equilibrium isotherm, kinetics, thermodynamics and regeneration studies. Arabian J. Sci. Eng. 43, 6011–6020 (2018)
Foo, K.Y.; Hameed, B.H.: Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 156, 2–10 (2010)
Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A.; Kooh, M.R.R.: Efficient adsorption of malachite green dye using Artocarpus odoratissimus leaves with artificial neural network modelling. Desalin. Water Treat. 101, 313–324 (2018)
Wehr, J.B.; Blamey, F.P.C.; Kopittke, P.M.; Menzies, N.W.: Comparative hydrolysis and sorption of Al and La onto plant cell wall material and pectic materials. Plant Soil 332, 319–330 (2010)
Oliveira, R.C.; Hammer, P.; Guibal, E.; Taulemesse, J.M.; Garcia Jr., O.: Characterization of metal–biomass interactions in the lanthanum(III) biosorption on Sargassum sp. using SEM/EDX, FTIR, and XPS: preliminary studies. Chem. Eng. J. 239, 381–391 (2014)
Marwani, H.M.; Albishri, H.M.; Jalal, T.A.; Soliman, E.M.: Study of isotherm and kinetic models of lanthanum adsorption on activated carbon loaded with recently synthesized Schiff’s base. Arab. J. Chem. 10, S1032–S1040 (2017)
Chen, Q.: Study on the adsorption of lanthanum(III) from aqueous solution by bamboo charcoal. J. Rare Earths 28, 125–131 (2010)
Lahiji, M.N.; Keshtkar, A.R.; Moosavian, M.A.: Adsorption of cerium and lanthanum from aqueous solutions by chitosan/polyvinyl alcohol/3-mercaptopropyltrimethoxy-silane beads in batch and fixed-bed systems. Part. Sci. Technol. 36, 340–350 (2016)
Ashour, R.M.; Abdel-khalek, A.A.; Ali, M.M.; Abdel-Magied, A.F.: Adsorption of La3+ and Gd3+ using magnetic iron oxide nanoparticles: mechanistic and kinetic study. Chem. Chem. Technol. 11, 101–108 (2016)
Koochaki-Mohammadpour, S.M.A.; Torab-Mostaedi, M.; Talebizadeh-Rafsanjani, A.; Naderi-Behdani, F.: Adsorption isotherm, kinetic, thermodynamic, and desorption studies of lanthanum and dysprosium on oxidized multiwalled carbon nanotubes. J. Dispers. Sci. Technol. 35, 244–254 (2014)
Hamadneh, I.; Alatawi, A.; Zalloum, R.; Albuqain, R.; Alsotari, S.; Khalili, F.I.; Al-Dujaili, A.H.: Comparison of Jordanian and standard diatomaceous earth as an adsorbent for removal of Sm(III) and Nd(III) from aqueous solution. Environ. Sci. Pollut. Res. 26, 20969–20980 (2019)
Behdani, F.N.; Rafsanjani, A.T.; Torab-Mostaedi, M.; Mohammadpour, S.M.A.K.: Adsorption ability of oxidized multiwalled carbon nanotubes towards aqueous Ce(III) and Sm(III). Korean J. Chem. Eng. 30, 448–455 (2013)
Zhang, L.; Ding, S.D.; Yang, T.; Zheng, G.C.: Adsorption behavior of rare earth elements using polyethyleneglycol (phosphomolybdate and tungstate) heteropolyacid sorbents in nitric solution. Hydrometallurgy 99, 109–114 (2009)
Galhoum, A.A.; Mahfouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E.: Diethylenetriamine-functionalized chitosan magnetic nano-based particles for the sorption of rare earth metal ions [Nd(III), Dy(III) and Yb(III)]. Cellulose 22, 2589–2605 (2015)
Hadjittofi, L.; Charalambous, S.; Pashalidis, I.: Removal of trivalent samarium from aqueous solutions by activated biochar derived from cactus fibres. J. Rare Earths 34, 99–104 (2016)
Sappidi, P.; Boda, A.; Ali, S.M.; Singh, J.K.: Adsorption of gadolinium (Gd3+) ions on the dibenzo crown ether (DBCE) and dicyclo hexano crown ether (DCHCE) grafted on the polystyrene surface: insights from all atom molecular dynamics simulations and experiments. J. Phys. Chem. C 123, 12276–12285 (2019)
Acknowledgements
The authors greatly acknowledge the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia (RISTEKDIKTI) for research grant award through PTUPT Grant No. NKB-1734/UN2.R3.1/HKP.05.00/2019 to E.K.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kusrini, E., Alhamid, M.I., Widiantoro, A.B. et al. Simultaneous Adsorption of Multi-lanthanides from Aqueous Silica Sand Solution Using Pectin–Activated Carbon Composite. Arab J Sci Eng 45, 7219–7230 (2020). https://doi.org/10.1007/s13369-020-04386-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04386-w