Abstract
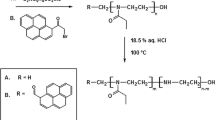
The use of polymer-based polyphosphazene is increasing in biomedical industry due to their degradable nature, and their specific role can further be tailored by substituting the chloro groups in the linear precursor with suitable nucleophiles. In this study, we aimed to synthesize a novel degradable polymer based on polyphosphazene by derivatizing the linear poly(dichlorophosphazene) precursor with diethyl amine and 2-(2-methoxyethoxy)ethanol. The structure of the synthesized polymer, poly[bis(2(2-methoxyethoxyethoxy diethylamino)phosphazene] (PMEEDEAP) was elucidated with 1H NMR and 31P NMR. The molar mass distribution and molecular weight of the synthesized polymers were assessed by employing GPC. The hydrolytic degradation, in vitro, of the polymer was carried out in phosphate-buffered saline (PBS) with pH ~ 7.0 and at 37 °C. The polymer showed a weight loss of 95% in 5 weeks. Current studies showed that the synthesized degradable polymer may further be subjected to in vivo studies and employed as a potential candidate for biomedical applications, i.e., controlled-drug delivery and tissue engineering. In addition, the experimental data were analyzed by graphical and statistical methods, and it was found that the weight loss of PMEEDEAP is linear function of time, i.e., \( w_{t} = - 1 \times 10^{-3} \;t_{{\text{hr}}} + 1.068. \) The value of the coefficient of determination (R2) is found to be 0.978, which indicates that the workability of the model is good.








Similar content being viewed by others
References
Akram, M.; Wang, L.; Yu, H.; Amer, W.A.; Khalid, H.; Abbasi, N.M.; Chen, Y.; Zain-Ul-, A.; Saleem, M.; Tong, R.: Polyphophazenes as anti-cancer drug carriers: from synthesis to application. Prog. Polym. Sci. 39, 1987–2010 (2014)
Teasdale, I.; Brüggemann, O.: Polyphosphazenes: multifunctional, biodegradable vehicles for drug and gene delivery. Polymers (Basel) 5, 161–187 (2013)
Amin, A.M.; Wang, L.; Wang, J.; Yu, H.; Huo, J.; Gao, J.; Xiao, A.: Recent research progress in the synthesis of polyphosphazenes and their applications. Des. Monomers Polym. 12, 357–375 (2009)
Amin, A.M.; Wang, L.; Wang, J.; Yu, H.; Gao, J.; Li, C.; Huo, J.; Amer, W.A.; Yan, G.; Ma, L.: Recent research progress in the synthesis of polyphosphazenes elastomers and their applications. J. Polym. Plast. Technol. Eng. 49, 1399–1405 (2010)
Amin, A.M.; Wang, L.; Wang, J.; Amer, W.A.; Huo, J.; Yu, H.; Gao, J.: Synthesis and characterization of poly[bis(resorcinol monobenzoate)phosphazenes], poly[bis(resorcinol monobenzoate diethylamino)phosphazene] and their self assembly behaviors. J. Inorg. Organomet. Polym. Mater. 21, 283–290 (2011)
Amin, A.M.; Wang, L.; Wang, J.; Yu, H.; Amer, W.A.; Gao, J.; Lei, Z.; Zhao, Y.; Huo, J.; Tai, Y.: Synthesis and characterization of poly[bis (p-oxybenzaldehyde diethylamino) phosphazenes], poly[bis(p-oxybenzaldehyde) phosphazene], poly[bis(di ethylamino)phosphazenes], and their self assembly behaviors. J. Macromol. Sci. Part A Pure Appl. Chem 48, 937–946 (2011)
Amin, A.M.; Wang, L.; Yu, H.; Amer, W.A.; Gao, J.; Huo, J.; Tai, Y.; Zhang, L.: Synthesis and characterization of poly[bis(ethyl salicylate)phosphazenes], poly[bis(ethyl salicylate diethylamino)phosphazenes] and their hydrolytic degradation. J. Inorg. Organomet. Polym. Mater. 22, 196–204 (2012)
Nichol, J.L.; Hotham, I.T.; Allcock, H.R.: Ethoxyphosphazene polymers and their hydrolytic behavior. Polym. Degrad. Stab. 109, 92–96 (2014)
Lee, C.; Su, J.; Kim, I.; Jun, H.; Hyung, T.; Taek, K.; Seong, E.; Choon, K.; Seok, Y.: Decanoic acid-modified glycol chitosan hydrogels containing tightly adsorbed palmityl-acylated exendin-4 as a long-acting sustained-release anti-diabetic system. Acta Biomater. 10, 812–820 (2014)
Song, S.; Sohn, Y.S.: Synthesis and hydrolytic properties of polyphosphazene/(diamine)platinum/saccharide conjugates. J. Control. Release 55, 161–170 (1998)
Oredein-McCoy, O.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Allcock, H.R.; Laurencin, C.T.: Novel factor-loaded polyphosphazene matrices: potential for driving angiogenesis. J. Microencapsul. 26, 544–555 (2009)
Potta, T.; Chun, C.; Song, S.C.: Chemically crosslinkable thermosensitive polyphosphazene gels as injectable materials for biomedical applications. Biomaterials 30, 6178–6192 (2009)
Bi, Y.M.; Gong, X.Y.; Wang, W.Z.; Yu, L.; Hu, M.Q.; Shao, L.D.: Synthesis and characterization of new biodegradable thermosensitive polyphosphazenes with lactic acid ester and methoxyethoxyethoxy side groups. Chin. Chem. Lett. 21, 237–241 (2010)
Brugmans, M.C.P.; Sӧntjens, S.H.M.; Cox, M.A.J.; Nandakumar, A.; Bosman, A.W.; Mes, T.; Janssen, H.M.; Bouten, C.V.C.; Baaijens, F.P.T.; Driessen-Mol, A.: Hydrolytic and oxidative degradation of electrospun supramolecular biomaterials: in vitro degradation pathways. Acta Biomater. 27, 21–31 (2015)
Nichol, J.L.; Allcock, H.R.: Polyphosphazenes with amino acid citronellol ester side groups for biomedical applications. Eur. Polym. J. 62, 214–221 (2015)
Allcock, H.R.: The expanding field of polyphosphazene high polymers. Dalton Trans. 45, 1856–1862 (2016)
Chih, H.; Mitsuru, U.; Ping, L.: Synthesis and characterization of polymer electrolytes based on cross-linked phenoxy-containing polyphosphazenes. J. Polym. Sci. Part A Polym. Chem. 54, 352–358 (2016)
Allcock, H.R.: Generation of structural diversity in polyphosphazenes. Appl. Organomet. Chem. Rev. 27(11), 620–629 (2013)
Chunying, M.; Xiao, Z.; Changguo, D.; Baojing, Z.; Chunhua, H.; Chao, L.; Renzhong, Q.: Water-soluble cationic polyphosphazenes grafted with cyclic polyamine and imidazole as an effective gene delivery vector. Bioconjug. Chem. 27(4), 1005–1012 (2016)
Jing, X.; Xiumei, Z.; Liyan, Q.: Polyphosphazene vesicles for co-delivery of doxorubicin and chloroquine with enhanced anticancer efficacy by drug resistance reversal. Int. J. Pharm. 498, 70–81 (2016)
Akram, M.; Haojie, Y.; Li, W.; Hamad, K.; Abbasi, N.M.; Yongsheng, Z.C.; Fujie, R.; Saleem, M.: Sustained release of hydrophilic drug from polyphosphazenes/poly(methyl methacrylate) based microspheres and their degradation study. Mater. Sci. Eng. C 58, 169–179 (2016)
Aoufi, D.; Serier, A.: Development and characterization of biodegradables packaging obtained from biopolymers mixture. J. Macromol. Sci. Part A Pure Appl. Chem. 55, 11–16 (2018)
Nor Faezah, A.; Suffian, M.; Annuar, M.: Functionalization of medium-chain-length poly(3-hydroxyalkanoates) as amphiphilic material by graft copolymerization with glycerol 1,3-diglycerolate diacrylate and its mechanism. J. Macromol. Sci. Part A Pure Appl. Chem. 55, 66–74 (2018)
Pradeep, K.D.; Diego, A.G.; Robert, J.M.: (Bio)polymeric hydrogels as therapeutic agents. J. Macromol. Sci. Part A Pure Appl. Chem. 44(12), 994–1003 (2011)
Vijayendra, K.; Bhavna, G.; Gaurav, K.; Mukesh, K.P.; Eric, A.; Virinder, S.P.; Jayant, K.; Watterson, A.C.: Novel PEGylated amphiphilic copolymers as nanocarriers for drug delivery: synthesis, characterization and curcumin encapsulation. J. Macromol. Sci. Part A Pure Appl. Chem. 47(12), 1154–1160 (2010)
Abd El-Mohdy, H.L.; El-Sayed, A.H.: Preparation of polyvinyl pyrrolidone-based hydrogels by radiation-induced crosslinking with potential application as wound dressing. J. Macromol. Sci. Part A Pure Appl. Chem. 45(12), 995–1002 (2008)
Helena, H.; Sandra, P.; Oliver, B.; Ian, T.: Polyphosphazene based star-branched and dendritic molecular brushes. Macromol. Rapid Commun. 37, 769–774 (2016)
Cuiyan, T.; Zhicheng, T.; Chen, C.; Zhongjing, L.; Tomasz, M.; Harry, R.A.: Synthesis and characterization of trifluoroethoxy polyphosphazenes containing polyhedral oligomeric silsesquioxane (POSS) side groups. Macromolecules 49, 1313–1320 (2016)
Tanzeela, G.S.; Shazia, K.; Amin, A.; Zulfiqar, A.; Shumaila, R.; Hajira, R.; Asmat, Z.; Shabbir, H.; Waqas, M.: Synthesis, characterization and hydrolytic degradation of p-Cresol substituted polyphosphazenes. Arab. J. Sci. Eng. (online) (2019). https://doi.org/10.1007/s13369-019-03952-1
Summe, U.R.; Wang, L.; Yu, H.; Haroon, M.; Elshaarani, T.; Kaleem, R.N.; Shah, F.; Amin, K.; Ahsan, N.; Xia, X.; Lisong, T.: Synthesis of polyphosphazene and preparation of microspheres from polyphosphazene blends with PMMA for drug combination therapy. J. Mater. Sci. 54, 745–764 (2019)
Magiri, R.; Mutwiri, G.; Wilson, H.L.: Recent advances in experimental polyphosphazene adjuvants and their mechanisms of action. Cell Tissue Res. 374(3), 465–471 (2018)
Yan, L.; Zhengping, Z.; Dingzhong, Y.; Yun, W.; Ying, D.; Yean, Z.: Introduction of amino groups into polyphosphazene framework supported on CNT and coated Fe3O4 nanoparticles for enhanced selective U(VI) adsorption. Appl. Surf. Sci. 466, 893–902 (2019)
Simge, M.Ö.; Yasemin, S.D.: One-pot synthesis and characterization of crosslinked polyphosphazene dopamine microspheres for controlled drug delivery applications. J. Macromol. Sci. Part A (2019). https://doi.org/10.1080/10601325.2019.1615838
Wei, H.H.; Pilar, S.G.; Esther, G.I.; Delyan, P.I.; Ruman, R.; Anna, M.G.; Noemi, C.; Cameron, A.; Marcos, G.F.: Structure-optimized interpolymer polyphosphazene complexes for effective gene delivery against glioblastoma. Adv. Ther. 2, 1800126 (2019)
Acknowledgements
Authors are grateful to the Higher Education Commission (HEC) for financial assistance of this project. Authors are also grateful to Dr Zafar Iqbal Zafar and Dr Anees for mathematical modeling and Dr Yar Muhammad, IRCBM COMSATS, Lahore, Pakistan, for their support regarding the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amin, A.M., Intisar, A., Hussain, H. et al. Synthesis, Characterization, Hydrolytic Degradation and Mathematical Modeling of Poly[bis(2(2-methoxyethoxyethoxy diethylamino)phosphazene]. Arab J Sci Eng 45, 241–247 (2020). https://doi.org/10.1007/s13369-019-04278-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-019-04278-8