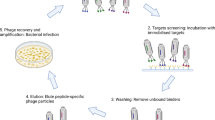
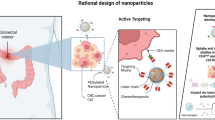
Abstract
Peptide-mediated targeting to colorectal cancer can increase selectivity and specificity of this cancer diagnosis acting as biomarkers. The present work aimed to select peptides using the phage display technique and associate the peptides with polymeric nanospheres in order to evaluate their cytotoxicity and selectivity during cell interaction with Caco-2 human colon tumor cell line. Two peptides identified by phage display (peptide-1 and peptide-2) were synthesized and exhibited purity higher than 84%. Poly(lactic acid)-block-polyethylene glycol nanospheres were prepared by nanoprecipitation and double emulsion methods in order to load the two peptides. Nanoparticles ranged in size from 114 to 150 nm and peptide encapsulation efficiency varied from 16 to 32%, depending on the methodology. No cytotoxic activity was observed towards Caco-2 tumor cell line, either free or loaded peptides in concentrations up to 3 μM at incubation times of 6 and 24 h, indicating safety as biomarkers. Fluorescein isothiocyanate–labeled peptides allowed evaluating selective interactions with Caco-2 cells, where peptide-1 entrapped in nanospheres showed greater intensity of co-localized cell fluorescence, in comparison to peptide-2. Peptide-1 loaded in nanospheres revealed promising to be investigated in further studies of selectivity with other human colon rectal cells as a potential biomarker.
Graphical abstract











Similar content being viewed by others
References
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Banerjee A, Pathak S, Subramanium VD, G.D., Murugesan R, Verma RS. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discov Today. 2017;22:1224–32 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1359644617302556.
Lee MW, Pourmorady JS, Laine L. Use of fecal occult blood testing as a diagnostic tool for clinical indications. Am J Gastroenterol. 2020;115:662–70.
Lee PY, Chin S-F, Low TY, Jamal R. Probing the colorectal cancer proteome for biomarkers: current status and perspectives. J Proteome. 2018;187:93–105 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1874391918302574.
Zhang A, Sun H, Yan G, Wang P, Han Y, Wang X. Metabolomics in diagnosis and biomarker discovery of colorectal cancer. Cancer Lett. 2014;345:17–20.
Schirripa M, Lenz H-J. Biomarker in colorectal cancer. Cancer J. 2016;22:156–64.
Tan A, Huang H, Zhang P, Li S. Network-based cancer precision medicine: a new emerging paradigm. Cancer Lett. 2019;458:39–45.
Wu C-H, Liu I-J, Lu R-M, Wu H-C. Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci. 2016;23:8.
Petropoulos K. Phage display. Methods Mol Biol. 2012;901:33–51.
Fang Y-M, Lin D-Q, Yao S-J. Review on biomimetic affinity chromatography with short peptide ligands and its application to protein purification. J Chromatogr A. 2018;1571:1–15.
Araste F, Abnous K, Hashemi M, Taghdisi SM, Ramezani M, Alibolandi M. Peptide-based targeted therapeutics: focus on cancer treatment. J Control Release. 2018;292:141–62. Available from. https://doi.org/10.1016/j.jconrel.2018.11.004.
Ma B, Niu F, Qu X, He W, Feng C, Wang S, Ouyang Z, Yan J, Wen Y, Xu D, Shao Y, Ma PX, Lu W. A tetrameric protein scaffold as a nano-carrier of antitumor peptides for cancer therapy. Biomaterials. 2019;204:1–12.
Scott DE, Bayly AR, Abell C, Skidmore J. Small molecules, big targets: drug discovery faces the protein–protein interaction challenge. Nat Rev Drug Discov. 2016;15:533–50.
Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81:136–47.
Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010;15:40–56.
Li Y, Clark KA, Tan Z. Methods for engineering therapeutic peptides. Chinese Chem Lett. 2018;29:1074–8. Available from. https://doi.org/10.1016/j.cclet.2018.05.027.
Batista P, Castro PM, Madureira AR, Sarmento B, Pintado M. Recent insights in the use of nanocarriers for the oral delivery of bioactive proteins and peptides. Peptides. 2018;101:112–23. Available from. https://doi.org/10.1016/j.peptides.2018.01.002.
Räder AFB, Reichart F, Weinmüller M, Kessler H. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorganic Med Chem. 2018;26:2766–73. Available from https://doi.org/10.1016/j.bmc.2017.08.031.
Martínez Rivas CJ, Tarhini M, Badri W, Miladi K, Greige-Gerges H, Nazari QA, Rodriguéz SAG, Román RA, Fessi H, Elaissari A. Nanoprecipitation process: from encapsulation to drug delivery. Int J Pharm. 2017;532:66–81. Available from https://doi.org/10.1016/j.ijpharm.2017.08.064.
Reix N, Parat A, Seyfritz E, Van Der Werf R, Epure V, Ebel N, Danicher L, Marchiori E, Jeandidier N, Pinget M, Frère Y, Sigrist S. In vitro uptake evaluation in Caco-2 cells and in vivo results in diabetic rats of insulin-loaded PLGA nanoparticles. Int J Pharm. 2012;437:213–20. Available from https://doi.org/10.1016/j.ijpharm.2012.08.024.
Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–37.
Gourdon B, Chemin C, Moreau A, Arnauld T, Delbos JM, Péan JM, Declèves X. Influence of PLA-PEG nanoparticles manufacturing process on intestinal transporter PepT1 targeting and oxytocin transport. Eur J Pharm Biopharm. 2018;129:122–33. Available from. https://doi.org/10.1016/j.ejpb.2018.05.022.
Gupta D, Kumar M, Tyagi P, Kapoor S, Tyagi A, Barman TK, et alKharbanda S, Kufe D, Singh H. Concomitant delivery of paclitaxel and NuBCP-9 peptide for synergistic enhancement of cancer therapy. Nanomedicine: Nanotechnology, Biol Med. 2018;14:1301–13. Available from. https://doi.org/10.1016/j.nano.2018.03.010.
Ding S, Serra CA, Vandamme TF, Yu W, Anton N. Double emulsions prepared by two–step emulsification: history, state-of-the-art and perspective. J Control Release. 2019;295:31–49.
Ye F, Zhao Y, El-Sayed R, Muhammed M, Hassan M. Advances in nanotechnology for cancer biomarkers. Nano Today. 2018;18:103–23.
Pound-Lana G, Rabanel J-M, Hildgen P, Mosqueira VCF. Functional polylactide via ring-opening copolymerisation with allyl, benzyl and propargyl glycidyl ethers. Eur Polym J. 2017;90:344–53. Available from. https://doi.org/10.1016/j.eurpolymj.2017.03.028.
Silva KT, Oliveira-Castro RA, Rodrigues VC, De Lima WG, Rodrigues CV, Castro-Borges W, Andrade MHG. DBT- and DBTO2-induced dysplasia and their associated proteomic alterations in the small intestines of Wistar rats. J Proteome Res. 2015;14:385–96. Available from. https://doi.org/10.1021/pr5009459.
Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:636–41.
Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:R1–4.
Quellec P, Gref R, Perrin L, Dellacherie E, Sommer F, Verbavatz JM, Alonso MJ. Protein encapsulation within polyethylene glycol-coated nanospheres. I. Physicochemical characterization. J Biomed Mater Res. 1998;42:45–54. Available from. https://doi.org/10.1002/(SICI)1097-4636(199810)42:1<45::AID-JBM7>3.0.CO;2-O
Egusquiaguirre SP, Manguán-García C, Perona R, Pedraz JL, Hernández RM, Igartua M. Development and validation of a rapid HPLC method for the quantification of GSE4 peptide in biodegradable PEI–PLGA nanoparticles. J Chromatogr B. 2014;972:95–101.
Meerovich I, Smith DD, Dash AK. Direct solid-phase peptide synthesis on chitosan microparticles for targeting tumor cells. J Drug Deliv Sci Technol. 2019;54:101288.
Śmiłowicz D, Metzler-Nolte N. Bioconjugates of Co(III) complexes with Schiff base ligands and cell penetrating peptides: solid phase synthesis, characterization and antiproliferative activity. J Inorg Biochem. 2020;206:111041.
Chan WC, White PD. Fmoc solid phase peptide synthesis: a practical approach. 1st ed. New York: Oxford University Press; 2000.
Lai MC, Topp EM. Solid-state chemical stability of proteins and peptides. J Pharm Sci. 1999;88:489–500.
De Boni S, Oberthür C, Hamburger M, Scriba GKE. Analysis of aspartyl peptide degradation products by high-performance liquid chromatography and high-performance liquid chromatography-mass spectrometry. J Chromatogr A. 2004;1022:95–102.
Antonioli P, Fortis F, Guerrier L, Rinalducci S, Zolla L, Righetti PG, Boschetti E. Capturing and amplifying impurities from purified recombinant monoclonal antibodies via peptide library beads: a proteomic study. Proteomics. 2007;7:1624–33.
Crucho CIC, Barros MT. Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater Sci Eng C. 2017;80:771–84.
Bilati U, Allémann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75.
Essa S, Rabanel JM, Hildgen P. Effect of polyethylene glycol (PEG) chain organization on the physicochemical properties of poly(d, l-lactide) (PLA) based nanoparticles. Eur J Pharm Biopharm. 2010;75:96–106.
Axson JL, Stark DI, Bondy AL, Capracotta SS, Maynard AD, Philbert MA, Bergin IL, Ault AP. Rapid kinetics of size and pH-dependent dissolution and aggregation of silver nanoparticles in simulated gastric fluid. J Phys Chem C. 2015;119:20632–41.
Buhecha MD, Lansley AB, Somavarapu S, Pannala AS. Development and characterization of PLA nanoparticles for pulmonary drug delivery: co-encapsulation of theophylline and budesonide, a hydrophilic and lipophilic drug. J Drug Deliv Sci Technol. 2019;53:101128.
Trindade IC, Pound-Lana G, Pereira DGS, de Oliveira LAM, Andrade MS, Vilela JMC, Postacchini B, Mosqueira VCF. Mechanisms of interaction of biodegradable polyester nanocapsules with non-phagocytic cells. Eur J Pharm Sci. 2018;124:89–104. Available from. https://doi.org/10.1016/j.ejps.2018.08.024
Oliveira LT, de Paula MA, Roatt BM, Garcia GM, Silva LSB, Reis AB, Paula CS, Vilela JMC, Andrade MS, Pound-Lana G, Mosqueira VCF. Impact of dose and surface features on plasmatic and liver concentrations of biodegradable polymeric nanocapsules. Eur J Pharm Sci. 2017;105:19–32. Available from. https://doi.org/10.1016/j.ejps.2017.04.017
Gaowa A, Horibe T, Kohno M, Sato K, Harada H, Hiraoka M, Tabata Y, Kawakami K. Combination of hybrid peptide with biodegradable gelatin hydrogel for controlled release and enhancement of anti-tumor activity in vivo. J Control Release. 2014;176:1–7.
Chen B, Yoo K, Xu W, Pan R, Han XX, Chen P. Characterization and evaluation of a peptide-based siRNA delivery system in vitro. Drug Deliv Transl Res. 2017;7:507–15.
Xiao YF, Jie MM, Li BS, Hu CJ, Xie R, Tang B, Yang SM. Peptide-based treatment: a promising cancer therapy. J Immunol Res. 2015;2015:1–13.
Bloch M, Kam Y, Yavin E, Moradov D, Nissan A, Ariel I, Rubinstein A. The relative roles of charge and a recognition peptide in luminal targeting of colorectal cancer by fluorescent polyacrylamide. Eur J Pharm Sci. 2012;47:904–13.
Kumari A, Singla R, Guliani A, Yadav SK. Biodegradable nanoparticles and their in vivo fate. In: Yadav SK, editor. Nanoscale materials in targeted drug delivery, theragnosis and tissue regeneration. Singapore: Springer Singapore; 2016. p. 21–39.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. We also thank the financial support from the following Brazilian funding agencies: CNPq grants (#310463/2015-7 and #481872/2013-2) and FAPEMIG grants (#APQ-02194-16, #APQ-02864-16, APQ-02576-18, and NANOBIOMG Network-RED-00007-14). VCFM is a productivity research fellow of CNPq. We acknowledge Raoni P. Siqueira for skillful help with cell culture and Prof. F.G. Araújo for giving access to AFM equipment in NanoLab/REDEMAT/UFOP acquired with financial support of CT-Infra FINEP/Brazil.
Author information
Authors and Affiliations
Contributions
Conceptualization: AS, VCFM, WCB, and KTSR. Methodology: AS, WCB, MHGA, YCPM, LRGF, EGL, ACFB, APMB, VCFM, and KTSR. Formal analysis and investigation: AS, WCB, APMB, VCFM, and KTSR. Original draft preparation: AS and KTSR. Writing—review and editing: AS, WCB, VCFM, and KTSR. Funding acquisition: KTSR, WCB, and VCFM. Resources: KTSR and VCFM. Supervision: VCFM and KTSR.
Corresponding author
Ethics declarations
Conflict of interest
A. Souza, W. Castro-Borges, M.H.G. Andrade, Y.C.P. Maia, L.R.G. Filho, E.G. Lanna, A.C.F. Brito, A.P.M. Barboza, V.C.F. Mosqueira, and K.T.S. Rubio declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, A., Castro-Borges, W., de Andrade, M.H.G. et al. PLA-PEG nanospheres decorated with phage display selected peptides as biomarkers for detection of human colorectal adenocarcinoma. Drug Deliv. and Transl. Res. 10, 1771–1787 (2020). https://doi.org/10.1007/s13346-020-00826-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00826-0