Abstract
Bone infections caused by Staphylococcus aureus are a major concern in medical care, particularly when associated with orthopedic-implant devices. The ability of the bacteria to form biofilms and their capacity to invade and persist within osteoblasts turn the infection eradication into a huge challenge. The reduction of antibiotic penetration through bacterial biofilms associated with the presence of persistent cells, ability to survive in the host, and high tolerance to antibiotics are some of the reasons for the difficult treatment of these infections. Effective therapeutic approaches are urgently needed. In this sense, lipid-based nanosystems, such as liposomes, have been investigated as an innovative and alternative strategy for the treatment of implant-associated S. aureus infections, due to their preferential accumulation at infected sites and interaction with S. aureus. This review highlights the recent advances on antibiotic-loaded liposome formulations both in vitro and in vivo and how the interaction with S. aureus biofilms may be improved by modulating the liposomal external surface.

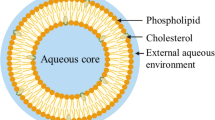
Graphical Abstract



Similar content being viewed by others
References
Ferreira M, Rzhepishevska O, Grenho L, Malheiros D, Gonçalves L, Almeida AJ, et al. Levofloxacin-loaded bone cement delivery system: highly effective against intracellular bacteria and Staphylococcus aureus biofilms. Int J Pharm. 2017;532:241–8.
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–61.
Bejon P, Robinson E. Bone and joint infection. Medicine. 2017;45:711–4.
Hibbitts A, O’Leary C. Emerging nanomedicine therapies to counter the rise of methicillin-resistant Staphylococcus aureus. Materials (Basel). 2018;11:321.
Bui LMG, Conlon BP, Kidd SP. Antibiotic tolerance and the alternative lifestyles of Staphylococcus aureus. Essays Biochem. 2017;61:71–9.
Conlon BP. Staphylococcus aureus chronic and relapsing infections: evidence of a role for persister cells. BioEssays. 2014;36:991–6.
Grassi L, Maisetta G, Esin S, Batoni G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front Microbiol. 2017;8:2409.
Sahukhal GS, Pandey S, Elasri MO. msaABCR operon is involved in persister cell formation in Staphylococcus aureus. BMC Microbiol. 2017;17:218.
Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, et al. ESCMID∗ guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21:S1–25.
Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–90.
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–32.
Alexander EH, Hudson MC. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl Microbiol Biotechnol. 2001;56:361–6.
Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–9.
Marriott I. Osteoblast responses to bacterial pathogens: a previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol Res. 2004;30:291–308.
Sinha B, Fraunholz M. Staphylococcus aureus host cell invasion and post-invasion events. Int J Med Microbiol. 2010;300:170–5.
Campoccia D, Montanaro L, Ravaioli S, Cangini I, Testoni F, Visai L, et al. New parameters to quantitatively express the invasiveness of bacterial strains from implant-related orthopaedic infections into osteoblast cells. Materials (Basel). 2018;11:E550.
Ellington JK, Harris M, Hudson MC, Vishin S, Webb LX, Sherertz R. Intracellular Staphylococcus aureus and antibiotic resistance: implications for treatment of staphylococcal osteomyelitis. J Orthop Res. 2006;24:87–93.
Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 2009;17:59–65.
Scherr TD, Hanke ML, Huang O, James DBA, Horswill AR, Bayles KW, et al. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio. 2015;6:25–7.
Lima ALL, Oliveira PR, Carvalho VC, Cimerman S, Savio E. Recommendations for the treatment of osteomyelitis. Braz J Infect Dis. 2014;18:526–34.
Fraimow HS. Systemic antimicrobial therapy in osteomyelitis. Semin Plast Surg. 2009;23:90–9.
Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. Penetration of antibacterials into bone pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet. 2009;48:89–124.
Lewis K. Platforms for antibiotic discovery. Nat Rev Drug Discov. 2013;12:371–87.
Akimoto Y, Kaneko K, Tamura T. Amoxicillin concentrations in serum, jaw cyst, and jawbone following a single oral administration. J Oral Maxillofac Surg. 1982;40:287–93.
Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54:393–407.
Pea F. Penetration of antibacterials into bone. Clin Pharmacokinet. 2009;48:125–7.
Bystedt H, Dahlbäck A, Dornbusch K, Nord CE. Concentrations of azidocillin, erythromycin, doxycycline and clindamycin in human mandibular bone. Int J Oral Surg. 1978;7:442–9.
Martin C, Alaya M, Mallet MN, Viviand X, Ennabli K, Said R, et al. Penetration of vancomycin in cardiac and mediastinal tissues in humans. Pathol Biol. 1994;42:520–4.
Massias L, Dubois C, De Lentdecker P, Brodaty O, Fischler M, Farinotti R. Penetration of vancomycin in uninfected sternal bone. Antimicrob Agents Chemother. 1992;36:2539–41.
Graziani AL, Lawson LA, Gibson GA, Steinberg MA, McGregor RR. Vancomycin concentrations in infected and noninfected human bone. Antimicrob Agents Chemother. 1988;32:1320–2.
Rimmele T. Diffusion of levofloxacin into bone and synovial tissues. J Antimicrob Chemother. 2004;53:533–5.
Baum H, Böttcher S, Abel R, Gerner H, Sonntag H-G. Tissue and serum concentrations of levofloxacin in orthopaedic patients. Int J Antimicrob Agents. 2001;18:335–40.
Traunmüller F, Schintler MV, Metzler J, Spendel S, Mauric O, Popovic M, et al. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J Antimicrob Chemother. 2010;65:1252–7.
Gomes D, Pereira M, Bettencourt AF. Osteomyelitis: an overview of antimicrobial therapy. Braz J Pharm Sci. 2013;49:13–27.
Cheng L, Renz N, Trampuz A. Management of periprosthetic joint infection. Kühn K-D, editor. Hip Pelvis Berlin. 2018;30:138–46.
Stengel D, Bauwens K, Sehouli J. Ekkernkamp a, Porzsolt F. Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect Dis. 2001;1:175–88.
Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125:353–64.
Cheng H, Chawla A, Yang Y, Li Y, Zhang J, Jang HL, et al. Development of nanomaterials for bone-targeted drug delivery. Drug Discov Today. 2017;22:1336–50.
Xiong MH, Bao Y, Yang XZ, Zhu YH, Wang J. Delivery of antibiotics with polymeric particles. Adv Drug Deliv Rev. 2014;78:63–76.
Nandi SK, Mukherjee P, Roy S, Kundu B, De DK, Basu D. Local antibiotic delivery systems for the treatment of osteomyelitis – a review. Mater Sci Eng C. 2009;29:2478–85.
Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–23.
Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–66.
Dos Santos Ramos MA, Da Silva P, Spósito L, De Toledo L, Bonifácio B, Rodero CF, et al. Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. Int J Nanomedicine. 2018;13:1179–213.
Rukavina Z, Vanić Ž. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics. 2016;8:E18.
Lewis G. Not all approved antibiotic-loaded PMMA bone cement brands are the same: ranking using the utility materials selection concept. J Mater Sci Mater Med. 2015;26:5388.
Bistolfi A, Massazza G, Verné E, Massè A, Deledda D, Ferraris S, et al. Antibiotic-loaded cement in orthopedic surgery: a review. ISRN Orthop. 2011;2011:1–8.
Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Jt Surg. 2006;88:2487–500.
Soares D, Leite P, Barreira P, Aido R, Sousa R. Antibiotic-loaded bone cement in total joint arthroplasty. Acta Orthop Belg. 2015;81:184–90.
Van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Infection of orthopedic implants and the use of antibiotic-loaded bone cements A review. Acta Orthop Scand. 2001;72:557–71.
Athans V, Veve MP, Davis SL. Trowels and tribulations: review of antimicrobial-impregnated bone cements in prosthetic joint surgery. Pharmacotherapy. 2017;37:1565–77.
Vaishya R, Chauhan M, Vaish A. Bone cement. J Clin Orthop Trauma. 2013;4:157–63.
Saleh KJ, El Othmani MM, Tzeng TH, Mihalko WM, Chambers MC, Grupp TM. Acrylic bone cement in total joint arthroplasty: a review. J Orthop Res. 2016;34:737–44.
Snoddy B, Jayasuriya AC. The use of nanomaterials to treat bone infections. Mater Sci Eng C. 2016;67:822–33.
Arora M, Chan EK, Gupta S, Diwan AD. Polymethylmethacrylate bone cements and additives: a review of the literature. World J Orthop. 2013;4:67–74.
Matos AC, Ribeiro IAC, Guedes RC, Pinto R, Vaz MA, Goncalves LM, et al. Key-properties outlook of a levofloxacin-loaded acrylic bone cement with improved antibiotic delivery. Int J Pharm. 2015;485:317–28.
Shi Z, Neoh KGG, Kang ETT, Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27:2440–9.
Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, et al. Nanoparticulate silver. A new antimicrobial substance for bone cement. Orthopade. 2004;33:885–92.
Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, et al. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–91.
Asli A, Brouillette E, Ster C, Ghinet MG, Brzezinski R, Lacasse P, et al. Antibiofilm and antibacterial effects of specific chitosan molecules on Staphylococcus aureus isolates associated with bovine mastitis. PLoS One. 2017;12:e0176988.
Li W-R, Xie X-B, Shi Q-S, Duan S-S, Ouyang Y-S, Chen Y-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals. 2011;24:135–41.
Zhang W, Lei G, Liu Y, Wang W, Song T, Fan J. Approach to osteomyelitis treatment with antibiotic loaded PMMA. Microb Pathog. 2017;102:42–4.
Nandi SK, Bandyopadhyay S, Das P, Samanta I, Mukherjee P, Roy S, et al. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol Adv. 2016;34:1305–17.
Bastari K, Arshath M, Ng ZHM, Chia JH, ZXD Y, Sana B, et al. A controlled release of antibiotics from calcium phosphate-coated poly(lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm. J Mater Sci Mater Med. 2014;25:747–57.
Ignjatović NL, Ninkov P, Sabetrasekh R, Uskoković DP. A novel nano drug delivery system based on tigecycline-loaded calciumphosphate coated with poly-dl-lactide-co-glycolide. J Mater Sci Mater Med. 2010;21:231–9.
Mifsud M, McNally M. Local delivery of antimicrobials in the treatment of bone infections. Orthop Traumatol. 2019;33:160–5.
Szurkowska K, Laskus A, Kolmas J. Hydroxyapatite-based materials for potential use in bone tissue infections. In: Thirumalai J, editor. InTech; 2018 pp. 109–35.
Kaya M, Şimşek-Kaya G, Gürsan N, Kireççi E, Dayı E, Gündoğdu B. Local treatment of chronic osteomyelitis with surgical debridement and tigecycline-impregnated calcium hydroxyapatite: an experimental study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:340–7.
Zhou Q, Wang T, Wang C, Wang Z, Yang Y, Li P, et al. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloids Surfaces A Physicochem Eng Asp. 2020;585:124081.
Zhang LG, Im O, Li J, Keidar M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int J Nanomedicine. 2012;7:2087.
Peng K-T, Chen C-F, Chu I-M, Li Y-M, Hsu W-H, Hsu RW-W, et al. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials. 2010;31:5227–36.
Moghadas-Sharif N, Fazly Bazzaz BS, Khameneh B, Malaekeh-Nikouei B. The effect of nanoliposomal formulations on Staphylococcus epidermidis biofilm. Drug Dev Ind Pharm. 2015;41:445–50.
Schiffelers RM, Storm G, Bakker-Woudenberg IA. Host factors influencing the preferential localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue. Pharm Res. 2001;18:780–7.
Gaspar M, Cruz A, Fraga A, Castro A, Cruz M, Pedrosa J. Developments on drug delivery systems for the treatment of mycobacterial infections. Curr Top Med Chem. 2008;8:579–91.
Gaspar MM, Calado S, Pereira J, Ferronha H, Correia I, Castro H, et al. Targeted delivery of paromomycin in murine infectious diseases through association to nano lipid systems. Nanomedicine. 2015;11:1851–60.
Gaspar MM, Radomska A, Gobbo OL, Bakowsky U, Radomski MW, Ehrhardt C. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J Aerosol Med Pulm Drug Deliv. 2012;25:310–8.
Cruz M, Simões S, Crow M, Martins M, Gaspar M. Formulation of nanoparticulate drug delivery systems (NPDDS) for macromolecules. In: Pathak Y, Thassu D, editors. Informa Healthcare USA I. New York: Drug Deliv nanoparticles Formul Charact; 2009. p. 35–49.
Xing H, Hwang K, Lu Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics. 2016;6:1336–52.
Rivero Berti I, Dell’Arciprete ML, Dittler ML, Miñan A, Fernández Lorenzo de Mele M, Gonzalez M. Delivery of fluorophores by calcium phosphate-coated nanoliposomes and interaction with Staphylococcus aureus biofilms. Colloids Surf B: Biointerfaces. 2016;142:214–22.
Vyas SP, Sihorkar V, Jain S. Mannosylated liposomes for bio-film targeting. Int J Pharm. 2007;330:6–13.
Dogbe MG, Mafilaza AY, Eleutério CV, Cabral-Marques H, Simões S, Gaspar MM. Pharmaceutical benefits of fluticasone propionate association to delivery systems: In vitro and in vivo evaluation. Pharmaceutics. 2019;11:E521.
Ranjan A, Pothayee N, Seleem MN, Tyler RD, Brenseke B, Sriranganathan N, et al. Antibacterial efficacy of core-shell nanostructures encapsulating gentamicin against an in vivo intracellular Salmonella model. Int J Nanomedicine. 2009;4:289–97.
Ranjan A, Pothayee N, Vadala TP, Seleem MN, Restis E, Sriranganathan N, et al. Efficacy of amphiphilic core-shell nanostructures encapsulating gentamicin in an in vitro Salmonella and Listeria intracellular infection model. Antimicrob Agents Chemother. 2010;54:3524–6.
Pinheiro M, Magalhães J, Reis S. Antibiotic interactions using liposomes as model lipid membranes. Chem Phys Lipids. 2019;222:36–46.
Kadry AA, Al-Suwayeh SA, Abd-Allah ARA, Bayomi MA. Treatment of experimental osteomyelitis by liposomal antibiotics. J Antimicrob Chemother. 2004;54:1103–8.
Dong D, Thomas N, Thierry B, Vreugde S, Prestidge CA, Wormald P-J. Distribution and inhibition of liposomes on Staphylococcus aureus and Pseudomonas aeruginosa biofilm. PLoS One. 2015;10:e0131806.
Forier K, Messiaen A-S, Raemdonck K, Nelis H, De Smedt S, Demeester J, et al. Probing the size limit for nanomedicine penetration into Burkholderia multivorans and Pseudomonas aeruginosa biofilms. J Control Release. 2014;195:21–8.
Onyeji CO, Nightingale CH, Marangos MN. Enhanced killing of methicillin-resistant Staphylococcus aureus in human macrophages by liposome-entrapped vancomycin and teicoplanin. Infection. 1994;22:338–42.
Sande L, Sanchez M, Montes J, Wolf AJ, Morgan MA, Omri A, et al. Liposomal encapsulation of vancomycin improves killing of methicillin-resistant Staphylococcus aureus in a murine infection model. J Antimicrob Chemother. 2012;67:2191–4.
Liu J, Wang Z, Li F, Gao J, Wang L, Huang G. Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: Characterization and evaluation. Asian J Pharm Sci. 2015;10:212–22.
Yang Z, Liu J, Gao J, Chen S, Huang G. Chitosan coated vancomycin hydrochloride liposomes: characterizations and evaluation. Int J Pharm. 2015;495:508–15.
Alshamsan A, Aleanizy FS, Badran M, Alqahtani FY, Alfassam H, Almalik A, et al. Exploring anti-MRSA activity of chitosan-coated liposomal dicloxacillin. J Microbiol Methods. 2019;156:23–8.
Zhu C-T, Xu Y-Q, Shi J, Li J, Ding J. Liposome combined porous β-TCP scaffold: preparation, characterization, and anti-biofilm activity. Drug Deliv. 2010;17:391–8.
Zhou T-H, Su M, Shang B-C, Ma T, Xu G-L, Li H-L, et al. Nano-hydroxyapatite/β-tricalcium phosphate ceramics scaffolds loaded with cationic liposomal ceftazidime: preparation, release characteristics in vitro and inhibition to Staphylococcus aureus biofilms. Drug Dev Ind Pharm. 2012;38:1298–304.
Ma T, Shang B-C, Tang H, Zhou T-H, Xu G-L, Li H-L, et al. Nano-hydroxyapatite/chitosan/konjac glucomannan scaffolds loaded with cationic liposomal vancomycin: preparation, in vitro release and activity against Staphylococcus aureus biofilms. J Biomater Sci Polym Ed. 2011;22:1669–81.
Hui T, Yongqing X, Tiane Z, Gang L, Yonggang Y, Muyao J, et al. Treatment of osteomyelitis by liposomal gentamicin-impregnated calcium sulfate. Arch Orthop Trauma Surg. 2009;129:1301–8.
Ayre WN, Birchall JC, Evans SL, Denyer SP. A novel liposomal drug delivery system for PMMA bone cements. J Biomed Mater Res Part B Appl Biomater. 2016;104:1510–24.
Liu X-M, Zhang Y, Chen F, Khutsishvili I, Fehringer EV, Marky LA, et al. Prevention of orthopedic device-associated osteomyelitis using oxacillin-containing biomineral-binding liposomes. Pharm Res. 2012;29:3169–79.
Laye C, McClements DJ, Weiss J. Formation of biopolymer-coated liposomes by electrostatic deposition of chitosan. J Food Sci. 2008;73:N7–15.
Stapleton M, Sawamoto K, Alméciga-Díaz CJ, Mackenzie WG, Mason RW, Orii T, et al. Development of bone targeting drugs. Int J Mol Sci. 2017;18:E1345.
Hengst V, Oussoren C, Kissel T, Storm G. Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int J Pharm. 2007;331:224–7.
Gaspar MM, Boerman OC, Laverman P, Corvo ML, Storm G, Cruz MEM. Enzymosomes with surface-exposed superoxide dismutase: in vivo behaviour and therapeutic activity in a model of adjuvant arthritis. J Control Release Netherlands. 2007;117:186–95.
Fielding RM. Liposomal Drug Delivery. Clin Pharmacokinet. 1991;21:155–64.
He H, Yuan D, Wu Y, Cao Y. Pharmacokinetics and pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics. 2019;11:110.
Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315.
Jøraholmen MW, Bhargava A, Julin K, Johannessen M, Škalko-Basnet N. The antimicrobial properties of chitosan can be tailored by formulation. Mar Drugs. 2020;18:1–15.
Mady MM, Darwish MM. Effect of chitosan coating on the characteristics of DPPC liposomes. J Adv Res. 2010;1:187–91.
Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Tanaka H, Inoue M, et al. Inhibition of vancomycin-induced nephrotoxicity by targeting superoxide dismutase to renal proximal tubule cells in the rat. Redox Rep. 2002;7:317–9.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42:S35–9 A.
Gaspar MM, Cruz A, Penha AF, Reymão J, Sousa AC, Eleutério CV, et al. Rifabutin encapsulated in liposomes exhibits increased therapeutic activity in a model of disseminated tuberculosis. Int J Antimicrob Agents. 2008;31:37–45.
Darley ESR, MacGowan AP. Antibiotic treatment of Gram-positive bone and joint infections. J Antimicrob Chemother. 2004;53:928–35.
Humphrey SJ, Mehta S, Seaber AV, Vail TP. Pharmacokinetics of a degradable drug delivery system in bone. Clin Orthop Relat Res. 1998;349:218–24.
Zylberberg C, Matosevic S. Bioengineered liposome–scaffold composites as therapeutic delivery systems. Ther Deliv. 2017;8:425–45.
Kendoff DO, Gehrke T, Stangenberg P, Frommelt L, Bösebeck H. Bioavailability of gentamicin and vancomycin released from an antibiotic containing bone cement in patients undergoing a septic one-stage total hip arthroplasty (THA) revision: a monocentric open clinical trial. HIP Int. 2016;26:90–6.
Luo S, Jiang T, Yang Y, Yang X, Zhao J. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskelet Disord. 2016;17:502.
Jiang N, Zhao X, Wang L, Lin Q, Hu Y, Yu B. Single-stage debridement with implantation of antibiotic-loaded calcium sulphate in 34 cases of localized calcaneal osteomyelitis. Acta Orthop. 2020;3674:1–7.
Funding
Fundação para a Ciência e a Tecnologia (FCT) of Portugal provided financial support (iMed.ULisboa, UID/DTP/04138/2019 and PTDC/MED-QUI/31721/2017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, M., Aguiar, S., Bettencourt, A. et al. Lipid-based nanosystems for targeting bone implant-associated infections: current approaches and future endeavors. Drug Deliv. and Transl. Res. 11, 72–85 (2021). https://doi.org/10.1007/s13346-020-00791-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00791-8