Abstract
Engineered immune cells offer a prime therapeutic alternate for some aggressive and frequently occurring malignancies like lung cancer. These therapies were reported to result in tumor regression and overall improvement in patient survival. However, studies also suggest that the presence of cancer cell-induced immune-suppressive microenvironment, off-target toxicity, and difficulty in concurrent imaging are some prime impendent in the success of these approaches. The present article reviews the need and significance of the currently available immune cell-based strategies for lung cancer therapeutics. It also showcases the utility of incorporating nanoengineered strategies and details the available formulations of nanocarriers. In last, it briefly discussed the existing methods for nanoparticle fuctionalization and challenges in translating basic research to the clinics.

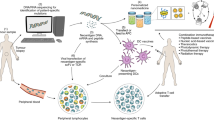
Graphical Abstract








Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240–2. https://doi.org/10.1097/JTO.0000000000000663.
Yatabe Y, Dacic S, Borczuk AC, et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol. 2019;14:377–407. https://doi.org/10.1016/j.jtho.2018.12.005.
Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 1856;2015:189–210. https://doi.org/10.1016/j.bbcan.2015.08.002.
Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–505. https://doi.org/10.1016/S1470-2045(19)30456-5.
Liao Y, Wang X, Zhong P, Yin G, Fan X, Huang C. A nomogram for the prediction of overall survival in patients with stage II and III non-small cell lung cancer using a population-based study. Oncol Lett. 2019;18:5905–16. https://doi.org/10.3892/ol.2019.10977.
Santarpia M, Giovannetti E, Rolfo C, Karachaliou N, González-Cao M, Altavilla G, et al. Recent developments in the use of immunotherapy in non-small cell lung cancer. Expert Rev Respir Med. 2016;10:781–98. https://doi.org/10.1080/1747634820161182866.
Kibirova A, Mattes MD, Smolkin M, Ma PC. The journey of an EGFR-mutant lung adenocarcinoma through erlotinib, osimertinib and ABCP immunotherapy regimens: sensitivity and resistance. Case Rep Oncol. 2019;12:765–76. https://doi.org/10.1159/000503417.
Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35:3449–57. https://doi.org/10.1200/JCO.2016.71.7629.
Frederickson AM, Arndorfer S, Zhang I, et al. Pembrolizumab plus chemotherapy for first-line treatment of metastatic nonsquamous non-small-cell lung cancer: a network meta-analysis. Immunotherapy. 2019;11:407–28. https://doi.org/10.2217/imt-2018-0193.
Boloker G, Wang C, Zhang J. Updated statistics of lung and bronchus cancer in United States (2018). J Thorac Dis. 2018;10:1158–61. https://doi.org/10.21037/jtd20190296.
Griswold CR, Kerrigan K, Patel SB. Combination of local ablative therapy and continuation of immune checkpoint inhibitor (ICI) therapy provides durable treatment response past oligometastatic progression in NSCLC: a case report. Case Rep Oncol. 2019;12:866–71. https://doi.org/10.1159/000504473.
Chen Y, Zhou Y, Tang L, Peng X, Jiang H, Wang G, et al. Immune-checkpoint inhibitors as the first line treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer. 2019;10:6261–8. https://doi.org/10.7150/jca.34677.
Spaas M, Lievens Y. Is the combination of immunotherapy and radiotherapy in non-small cell lung cancer a feasible and effective approach? Front Med (Lausanne). 2019;6:244. https://doi.org/10.3389/fmed.2019.00244.
Xu Y, Wan B, Chen X, Zhan P, Zhao Y, Zhang T, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res. 2019;8:413–28. https://doi.org/10.21037/tlcr.2019.08.09.
Ferro S, Huber V, Rivoltini L. Mechanisms of tumor immunotherapy, with a focus on thoracic cancers. J Thorac Dis. 2018;10:4619–31. https://doi.org/10.21037/jtd.2018.07.30.
Bhargava A, Mishra DK, Jain SK, Srivastava RK, Lohiya NK, Mishra PK. Comparative assessment of lipid based nano-carrier systems for dendritic cell based targeting of tumor re-initiating cells in gynecological cancers. Mol Immunol. 2016;79:98–112. https://doi.org/10.1016/jmolimm201610003.
Bhargava A, Mishra D, Banerjee S, Mishra PK. Dendritic cell engineering for tumor immunotherapy: from biology to clinical translation. Immunotherapy. 2012;4:703–18. https://doi.org/10.2217/imt1240.
Bhargava A, Mishra D, Khan S, Varshney SK, Banerjee S, Mishra PK. Assessment of tumor antigen-loaded solid lipid NPs as an efficient delivery system for dendritic cell engineering. Nanomedicine (London). 2013;8:1067–84. https://doi.org/10.2217/nnm12164.
Duong CP, Yong CS, Kershaw MH, Slaney CY, Darcy PK. Cancer immunotherapy utilizing gene-modified T-cells: from the bench to the clinic. Mol Immunol. 2015;67:46–57. https://doi.org/10.1016/jmolimm2014.12009.
Met Ö, Jensen KM, Chamberlain CA, Donia M, Svane IM. Principles of adoptive T-cell therapy in cancer. Semin Immunopathol. 2019;41:49–58. https://doi.org/10.1007/s00281-018-0703-z.
Yee C. Adoptive T-cell therapy: points to consider. Curr Opin Immunol. 2018;51:197–203. https://doi.org/10.1016/jcoi201804007.
Sharpe M, Mount N. Genetically modified T-cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8:337–50. https://doi.org/10.1242/dmm018036.
Chen D, Yang J. Development of novel antigen receptors for CAR T-cell therapy directed toward solid malignancies. Transl Res. 2017;187:11–21. https://doi.org/10.1016/jtrsl2017.05.006.
Wang X, Riviere I. Manufacture of tumor- and virus-specific T lymphocytes for adoptive cell therapies. Cancer Gene Ther. 2015;22:85–94. https://doi.org/10.1038/cgt.2014.81.
Spear TT, Nagato K, Nishimura MI. Strategies to genetically engineer T-cells for cancer immunotherapy. Cancer Immunol Immunother. 2016;65:631–49. https://doi.org/10.1007/s00262-016-1842-5.
Zeltsman M, Dozier J, McGee E, Ngai D, Adusumilli PS. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Transl Res. 2017;187:1–10. https://doi.org/10.1016/jtrsl.2017.04.004.
Lichtman EI, Dotti G. Chimeric antigen receptor T-cells for B-cell malignancies. Transl Res. 2017;187:59–82. https://doi.org/10.1016/jtrsl.2017.06.011.
Townsend MH, Shrestha G, Robison RA, O'Neill KL. The expansion of targetable biomarkers for CAR T-cell therapy. J Exp Clin Cancer Res. 2018;37:163. https://doi.org/10.1186/s13046-018-0817-0.
Jung IY, Lee J. Unleashing the therapeutic potential of CAR-T-cell therapy using gene-editing technologies. Mol Cell. 2018;41:717–23. https://doi.org/10.14348/molcells20180242.
Gacerez AT, Hua CK, Ackerman ME, Sentman CL. Chimeric antigen receptors with human scFvs preferentially induce T-cell anti-tumor activity against tumors with high B7H6 expression. Cancer Immunol Immunother. 2018;67:749–59. https://doi.org/10.1007/s00262-018-2124-1.
Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T-cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36:847–56. https://doi.org/10.1038/nbt.4195.
Bigley AB, Simpson RJ. NK-cells and exercise: implications for cancer immunotherapy and survivorship. Discov Med. 2015;19:433–45.
Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26:161–72. https://doi.org/10.1016/jsmim201402.002.
Dianat-Moghadam H, Rokni M, Marofi F, Panahi Y, Yousefi M. Natural killer cell-based immunotherapy: from transplantation toward targeting cancer stem cells. J Cell Physiol. 2018;234:259–73. https://doi.org/10.1002/jcp.26878.
Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:91. https://doi.org/10.3389/fimmu2016.00091.
Sakamoto N, Ishikawa T, Kokura S, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. https://doi.org/10.1186/s12967-015-0632-8.
Delgado D, Webster DE, DeSantes KB, Durkin ET, Shaaban AF. KIR receptor-ligand incompatibility predicts killing of osteosarcoma cell lines by allogeneic NK cells. Pediatr Blood Cancer. 2010;55:1300–5. https://doi.org/10.1002/pbc.22665.
Ichise H, Nagano S, Maeda T, et al. NK cell alloreactivity against KIR-ligand-mismatched HLA-haploidentical tissue derived from HLA haplotype-homozygous iPSCs. Stem Cell Reports. 2017;9:853–67. https://doi.org/10.1016/jstemcr.2017.07.020.
Murakami T, Nakazawa T, Natsume A, et al. Novel human NK cell line carrying CAR targeting EGFRvIII induces antitumor effects in glioblastoma cells. Anticancer Res. 2018;38:5049–56. https://doi.org/10.21873/anticanres12824.
Tang X, Yang L, Li Z, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8:1083–9.
Bollino D, Webb TJ. Chimeric antigen receptor-engineered natural killer and natural killer T cells for cancer immunotherapy. Transl Res. 2017;187:32–43. https://doi.org/10.1016/jtrsl2017.06.003.
Oelsner S, Friede ME, Zhang C, et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy. 2017;19:235–49. https://doi.org/10.1016/jjcyt2016.10.009.
Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–9. https://doi.org/10.1007/s00262-010-0904-3.
Schonfeld K, Sahm C, Zhang C, et al. Selective inhibition of tumor growth by clonal NK-cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23:330–8. https://doi.org/10.1038/mt2014219.
Sakamoto M, Nakajima J, Murakawa T, et al. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded gammadelta T cells: a phase I clinical study. J Immunother. 2011;34:202–11. https://doi.org/10.1097/CJI0b013e318207ecfb.
Nakajima J, Murakawa T, Fukami T, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T-cells. Eur J Cardiothorac Surg. 2010;37:1191–7. https://doi.org/10.1016/jejcts.2009.11.051.
Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T-cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–20.
Thistlethwaite FC, Gilham DE, Guest RD, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T-cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017;66:1425–36. https://doi.org/10.1007/s00262-017-2034-7.
Motohashi S, Ishikawa A, Ishikawa E, et al. A phase I study of in vitro expanded natural killer T-cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–86. https://doi.org/10.1158/1078-0432CCR-06-0114.
Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–70. https://doi.org/10.1016/jjcyt2013.06.017.
Perroud MW, Honma HN Jr, Barbeiro AS, et al. Mature autologous dendritic cell vaccines in advanced non-small cell lung cancer: a phase I pilot study. J Exp Clin Cancer Res. 2011;30:65. https://doi.org/10.1186/1756-9966-30-65.
Um SJ, Choi YJ, Shin HJ, et al. Phase I study of autologous dendritic cell tumor vaccine in patients with non-small cell lung cancer. Lung Cancer. 2010;70:188–94. https://doi.org/10.1016/jlungcan.2010.02.006.
Hu RH, Shi SB, Qi JL, et al. Pemetrexed plus dendritic cells as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer who had treatment with TKI. Med Oncol. 2014;31:63. https://doi.org/10.1007/s12032-014-0063-z.
Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60:1497–502. https://doi.org/10.1007/s00262-011-1060-0.
Zhu XP, Xu YH, Zhou J, Pan XF. A clinical study evaluating dendritic and cytokine-induced killer cells combined with concurrent radiochemotherapy for stage IIIB non-small cell lung cancer. Genet Mol Res. 2015;14:10228–35. https://doi.org/10.4238/2015.
Ma J, Liu H, Wang X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer. J Tradit Chin Med. 2014;34:641–5.
Kimura H, Matsui Y, Ishikawa A, Nakajima T, Iizasa T. Randomized controlled phase III trial of adjuvant chemoimmunotherapy with activated cytotoxic T-cells and dendritic cells from regional lymph nodes of patients with lung cancer. Cancer Immunol Immunother. 2018;67:1231–8. https://doi.org/10.1007/s00262-018-2180-6.
Kimura H, Matsui Y, Ishikawa A, Nakajima T, Yoshino M, Sakairi Y. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T-cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol Immunother. 2015;64:51–9. https://doi.org/10.1007/s00262-014-1613.
Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–18. https://doi.org/10.1007/s00262-013-1396-8.
Saka H, Kitagawa C, Ichinose Y, et al. A randomized phase II study to assess the effect of adjuvant immunotherapy using alpha-GalCer-pulsed dendritic cells in the patients with completely resected stage II-IIIA non-small cell lung cancer: study protocol for a randomized controlled trial. Trials. 2017;18:429.
Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51. https://doi.org/10.1016/jcoi.2015.12.007.
Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol. 2013;190:2001–8. https://doi.org/10.4049/jimmunol.1201379.
Ward-Hartstonge KA, Kemp RA. Regulatory T-cell heterogeneity and the cancer immune response. Clin Transl Immunology. 2017;6:e154. https://doi.org/10.1038/cti.2017.43.
Kim HR, Park HJ, Son J, et al. Tumor microenvironment dictates regulatory T cell phenotype: Upregulated immune checkpoints reinforce suppressive function. J Immunother Cancer. 2019;7:339. https://doi.org/10.1186/s40425-019-0785-8.
Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid derived suppressor cells interactions with natural killer cells and pro-angiogenic activities: roles in tumor progression. Front Immunol. 2019;10:771. https://doi.org/10.3389/fimmu.2019.00771.
Choi JN, Sun EG, Cho SH. IL-12 enhances immune response by modulation of myeloid derived suppressor cells in tumor microenvironment. Chonnam Med J. 2019;55:31–9. https://doi.org/10.4068/cmj.2019.55.1.31.
Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–44. https://doi.org/10.4049/jimmunol.0804253.
Rodriguez PC, Ochoa AC, Al-Khami AA. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front Immunol. 2017;8:93. https://doi.org/10.3389/fimmu.2017.00093.
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78. https://doi.org/10.1186/s12929-019-0568-z.
Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. https://doi.org/10.1038/cddis2015162.
Kroemer G, Galluzzi L. Immunotherapy of hematological cancers: PD-1 blockade for the treatment of Hodgkin’s lymphoma. Oncoimmunology. 2015;4:e1008853. https://doi.org/10.1080/2162402X20151008853.
Kudinov AE, Deneka A, Nikonova AS, et al. Musashi-2 (MSI2) supports TGF-beta signaling and inhibits claudins to promote non-small cell lung cancer (NSCLC) metastasis. Proc Natl Acad Sci U S A. 2016;113:6955–60. https://doi.org/10.1073/pnas.1513616113.
Wu DM, Deng SH, Liu T, Han R, Zhang T, Xu Y. TGF-beta-mediated exosomal lnc-MMP2-2 regulates migration and invasion of lung cancer cells to the vasculature by promoting MMP2 expression. Cancer Med. 2018;7:5118–29. https://doi.org/10.1002/cam4.1758.
Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. https://doi.org/10.1146/annurev-immunol-032713-120257.
Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med (Berl). 2016;94:509–22. https://doi.org/10.1007/s00109-015-1376-x.
Abediankenari S, Ghasemi M. Generation of immune inhibitory dendritic cells and CD4+T regulatory cells inducing by TGF-beta. Iran J Allergy Asthma Immunol. 2009;8:25–30.
Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–51. https://doi.org/10.1189/jlb0705358.
Sun Y, Jin X, Liu X, et al. MicroRNA let-7i regulates dendritic cells maturation targeting interleukin-10 via the Janus kinase 1-signal transducer and activator of transcription 3 signal pathway subsequently induces prolonged cardiac allograft survival in rats. J Heart Lung Transplant. 2016;35:378–88. https://doi.org/10.1016/jhealun2015.10.041.
Gil-Torregrosa BC, Lennon-Dumenil AM, Kessler B, et al. Control of cross-presentation during dendritic cell maturation. Eur J Immunol. 2004;34:398–407. https://doi.org/10.1002/eji200324508.
Huang FP, Chen YX, To CK. Guiding the “misguided” - functional conditioning of dendritic cells for the DC-based immunotherapy against tumors. Eur J Immunol. 2011;41:18–25. https://doi.org/10.1002/eji201040543.
Liu B, Qu L, Yan S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015;15:106. https://doi.org/10.1186/s12935-015-0260-7.
van Baren N, Van den Eynde BJ. Tumoral immune resistance mediated by enzymes that degrade tryptophan. Cancer Immunol Res. 2015;3:978–85. https://doi.org/10.1158/2326-6066.CIR-15-0095.
Trabanelli S, Lecciso M, Salvestrini V, et al. PGE2-induced IDO1 inhibits the capacity of fully mature DCs to elicit an in vitro antileukemic immune response. J Immunol Res. 2015;2015:253191. https://doi.org/10.1155/2015/253191.
Trabanelli S, Ocadlikova D, Ciciarello M, et al. The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells. J Immunol. 2014;192:1231–40. https://doi.org/10.4049/jimmunol.1300720.
Hack K, Reilly L, Proby C, Fleming C, Leigh I, Foerster J. Wnt5a inhibits the CpG oligodeoxynucleotide-triggered activation of human plasmacytoid dendritic cells. Clin Exp Dermatol. 2012;37:557–61. https://doi.org/10.1111/j1365.2230.201204362.x.
Fang Y, Kang Y, Zou H, et al. Beta-elemene attenuates macrophage activation and proinflammatory factor production via crosstalk with Wnt/beta-catenin signaling pathway. Fitoterapia. 2018;124:92–102. https://doi.org/10.1016/jfitote.2017.10.015.
Valencia J, Hernandez-Lopez C, Martinez VG, et al. Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J Immunol. 2011;187:4129–39. https://doi.org/10.4049/jimmunol.1101243.
Hong Y, Manoharan I, Suryawanshi A, et al. Beta-catenin promotes regulatory T-cell responses in tumors by inducing vitamin a metabolism in dendritic cells. Cancer Res. 2015;75:656–65. https://doi.org/10.1158/0008-5472.CAN-14-2377.
Holtzhausen A, Zhao F, Evans KS, et al. Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunol Res. 2015;3:1082–95. https://doi.org/10.1158/2326-6066.CIR-14-0167.
Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41:41–8. https://doi.org/10.1007/s00281-018-0702-0.
Amreddy N, Babu A, Muralidharan R, Munshi A, Ramesh R. Polymeric nanoparticle-mediated gene delivery for lung cancer treatment. Top Curr Chem (Cham). 2017;375:35. https://doi.org/10.1007/s41061-017-0128-5.
Mangal S, Gao W, Li T, Zhou QT. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharmacol Sin. 2017;38:782–97. https://doi.org/10.1038/aps.2017.34.
Jain D, Jain V, Singh R. Novel antigen delivery technologies: a review. Drug Deliv Transl Res. 2011;1:103–12. https://doi.org/10.1007/s13346-011-0014-6.
Chen H, Zeng Y, Liu W, Zhao S, Wu J, Du Y. Multifaceted applications of nanomaterials in cell engineering and therapy. Biotechnol Adv. 2013;31:638–53.
Swetha KL, Roy A. Tumor heterogeneity and nanoparticle-mediated tumor targeting: the importance of delivery system personalization. Drug Deliv Transl Res. 2018;8:1508–26. https://doi.org/10.1007/s13346-018-0578-5.
Yu W, Liu C, Liu Y, Zhang N, Xu W. Mannan-modified solid lipid NPs for targeted gene delivery to alveolar macrophages. Pharm Res. 2010;27:1584–96. https://doi.org/10.1007/s11095-010-0149-z.
Kaur A, Jyoti K, Rai S, et al. Tetanus toxoid-loaded cationic non-aggregated nanostructured lipid particles triggered strong humoral and cellular immune responses. J Microencapsul. 2016;33:263–73. https://doi.org/10.3109/02652048.2016.1169324.
Li P, Zhou J, Huang P, et al. Self-assembled PEG-b-PDPA-b-PGEM copolymer NPs as protein antigen delivery vehicles to dendritic cells: preparation, characterization and cellular uptake. Regen Biomater. 2017;4:11–20. https://doi.org/10.1093/rb/rbw044.
Chen X, Liu Y, Wang L, et al. Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg. Mol Pharm. 2014;11:1772–84. https://doi.org/10.1021/mp400597z.
Schmid D, Park CG, Hartl CA, et al. T-cell-targeting NPs focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8:1747. https://doi.org/10.1038/s41467-017-01830-8.
Duong HTT, Kim NW, Thambi T, et al. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J Control Release. 2018;269:225–34. https://doi.org/10.1016/jjconrel.2017.11.025.
Brinkhoff B, Ostroumov D, Heemcke J, et al. Microsphere priming facilitates induction of potent therapeutic T-cell immune responses against autochthonous liver cancers. Eur J Immunol. 2014;44:1213–24. https://doi.org/10.1002/eji.201343794.
Li HS, Singh B, Park TE, et al. Mannan-decorated thiolated Eudragit microspheres for targeting antigen presenting cells via nasal vaccination. Eur J Immunol. 2015;80:16–25. https://doi.org/10.1016/jejps201509014.
Vang KB, Safina I, Darrigues E, et al. Modifying dendritic cell activation with plasmonic nano vectors. Sci Rep. 2017;7:5513. https://doi.org/10.1038/s41598-017-04459-1.
Liang R, Xie J, Li J, et al. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. 2017;149:41–50. https://doi.org/10.1016/jbiomaterials.2017.09.029.
Climent N, Garcia I, Marradi M, et al. Loading dendritic cells with gold NPs (GNPs) bearing HIV-peptides and mannosides enhance HIV-specific T-cell responses. Nanomedicine. 2018;14:339–51. https://doi.org/10.1016/jnano.2017.11.009.
Nam J, Son S, Moon JJ. Adjuvant-loaded spiky gold NPs for activation of innate immune cells. Cell Mol Bioeng. 2017;10:341–55. https://doi.org/10.1007/s12195-017-0505-8.
Zhao Y, Zhao X, Cheng Y, Guo X, Yuan W. Iron oxide NPs-based vaccine delivery for cancer treatment. Mol Pharm. 2018;15:1791–9. https://doi.org/10.1021/acsmolpharmaceut.7b01103.
Zhang P, Chiu YC, Tostanoski LH, Jewell CM. Polyelectrolyte multilayers assembled entirely from immune signals on gold NP templates promote antigen-specific T-cell response. ACS Nano. 2015;9:6465–77. https://doi.org/10.1021/acsnano.5b02153.
Wu L, Wang Z, Zhang Y, et al. In situ probing of cell–cell communications with surface-enhanced Raman scattering (SERS) nanoprobes and microfluidic networks for screening of immunotherapeutic drugs. Nano Res. 2017;10:584–94. https://doi.org/10.1007/s12274-016-1316-2.
Chiang CS, Lin YJ, Lee R, et al. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumor-localized immunotherapy. Nat Nanotechnol. 2018;13:746–54. https://doi.org/10.1038/s41565-018-0146-7.
Nawwab Al-Deen FM, Selomulya C, Kong YY, et al. Design of magnetic polyplexes taken up efficiently by dendritic cell for enhanced DNA vaccine delivery. Gene Ther. 2014;21:212–8. https://doi.org/10.1038/gt2013.77.
Cho NH, Cheong TC, Min JH, et al. A multifunctional core-shell NP for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6:675–82. https://doi.org/10.1038/nnano.2011.149.
Zilio S, Vella JL, De la Fuente AC, et al. 4PD functionalized dendrimers: a flexible tool for in vivo gene silencing of tumor-educated myeloid cells. J Immunol. 2017;198:4166–77. https://doi.org/10.4049/jimmunol1600833.
Chahal JS, Khan OF, Cooper CL, et al. Dendrimer-RNA NPs generate protective immunity against lethal Ebola, H1N1 influenza, and toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci U S A. 2016;113:E4133–42. https://doi.org/10.1073/pnas.1600299113.
Aldinucci A, Turco A, Biagioli T, et al. Carbon nanotube scaffolds instruct human dendritic cells: modulating immune responses by contacts at the nanoscale. Nano Lett. 2013;13:6098–105. https://doi.org/10.1021/nl403396e.
Sinha A, Cha BG, Choi Y, et al. Carbohydrate-functionalized rGO as an effective cancer vaccine for stimulating antigen-specific cytotoxic T-cells and inhibiting tumor growth. Chem Mater. 2017;29:6883–92. https://doi.org/10.1021/acs.chemmater.7b02197.
Tao Y, Ju E, Ren J, Qu X. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials. 2014;35:9963–71. https://doi.org/10.1016/jbiomaterials.201408036.
Chiu YC, Gammon JM, Andorko JI, Tostanoski LH, Jewell CM. Modular vaccine design using carrier-free capsules assembled from polyionic immune signals. ACS Biomater Sci Eng. 2015;1:1200–5. https://doi.org/10.1021/acsbiomaterials5b00375.
Lee HY, Mohammed KA, Nasreen N. Nanoparticle-based targeted gene therapy for lung cancer. Am J Cancer Res. 2016;6:1118–34.
Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–26. https://doi.org/10.1016/j.addr.2008.08.005.
Karra N, Benita S. The ligand nanoparticle conjugation approach for targeted cancer therapy. Curr Drug Metab. 2012;13:22–41. https://doi.org/10.2174/138920012798356899.
Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769–84. https://doi.org/10.1007/s00432-014-1767-3.
Babu A, Templeton AK, Munshi A, Ramesh R. Nanoparticle-based drug delivery for therapy of lung cancer: progress and challenges. J Nanomater. 2013;863951:11. https://doi.org/10.1155/2013/863951.
Park W, Heo YJ, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:24. https://doi.org/10.1186/s40824-018-0133-y eCollection 2018.
Velpurisiva P, Gad A, Piel B, Jadia R, Rai P. Nanoparticle design strategies for effective cancer immunotherapy. J Biomed (Syd). 2017;2:64–77. https://doi.org/10.7150/jbm.18877.
Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials. 2014;35:8186–96. https://doi.org/10.1016/jbiomaterials201405077.
Bhargava A, Mishra D, Banerjee S, Mishra PK. Engineered dendritic cells for gastrointestinal tumor immunotherapy: opportunities in translational research. J Drug Target. 2013;21:126–36. https://doi.org/10.3109/1061186X.2012.731069.
Nascimento TL, Hillaireau H, Vergnaud J, Fattal E. Lipid-based nanosystems for CD44 targeting in cancer treatment: recent significant advances, ongoing challenges and unmet needs. Nanomedicine (London). 2016;11:1865–87. https://doi.org/10.2217/nnm-2016-5000.
Dhas NL, Kudarha RR, Acharya NS, Acharya SR. Polymeric immunonps mediated cancer therapy: versatile nanocarriers for cell-specific cargo delivery. Crit Rev Ther Drug Carrier Syst. 2018;35:1–64. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2017018714.
Kosmides AK, Necochea K, Hickey JW, Schneck JP. Separating T-cell targeting components onto magnetically clustered NPs boosts activation. Nano Lett. 2018;18:1916–24. https://doi.org/10.1021/acs.nanolett7b05284.
Muthukumarasamyvel T, Rajendran G, Santhana Panneer D, Kasthuri J, Kathiravan K, Rajendiran N. Role of surface hydrophobicity of dicationic amphiphile-stabilized gold NPs on A549 lung cancer cells. ACS Omega. 2017;2:3527–38. https://doi.org/10.1021/acsomega.7b00353.
Dai L, Yu Y, Luo Z, et al. Photosensitizer enhanced disassembly of amphiphilic micelle for ROS-response targeted tumor therapy in vivo. Biomaterials. 2016;104:1–17. https://doi.org/10.1016/jbiomaterials.201607002.
Saleem J, Wang L, Chen C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv Healthc Mater. 2018;7:e1800525. https://doi.org/10.1002/adhm201800525.
Sanz-Ortega L, Rojas JM, Portilla Y, Pérez-Yagüe S, Barber DF. Magnetic nanoparticles attached to the NK cell surface for tumor targeting in adoptive transfer therapies does not affect cellular effector functions. Front Immunol. 2019;10:2073. https://doi.org/10.3389/fimmu.2019.02073.
Mishra D, Mishra PK, Dubey V, Dabadghao S, Jain NK. Evaluation of uptake and generation of immune response by murine dendritic cells pulsed with hepatitis B surface antigen-loaded elastic liposomes. Vaccine. 2007;25:6939–44. https://doi.org/10.1016/jvaccine200706055.
Mizrahy S, Hazan-Halevy I, Landesman-Milo D, Ng BD, Peer D. Advanced strategies in immune modulation of cancer using lipid-based NPs. Front Immunol. 2017;8:69. https://doi.org/10.3389/fimmu.2017.00069.
Mishra DK, Shandilya R, Mishra PK. Lipid based nanocarriers: a translational perspective. Nanomedicine. 2018;14:2023–50. https://doi.org/10.1016/jnano201805021.
Jyoti K, Kaur K, Pandey RS, Jain UK, Chandra R, Madan J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: in vitro and in vivo studies. Colloid Interface Sci. 2015;445:219–30.
Sun L, Wan K, Hu X, et al. Functional nanoemulsion-hybrid lipid nanocarriers enhance the bioavailability and anti-cancer activity of lipophilic diferuloylmethane. Nanotechnology. 2016;27:085102.
Wan K, Sun L, Hu X, et al. Novel nanoemulsion based lipid nanosystems for favorable in vitro and in vivo characteristics of curcumin. Int J Pharm. 2016;504:80–8.
Asmawi AA, Salim N, Ngan CL, et al. Excipient selection and aerodynamic characterization of nebulized lipid-based nanoemulsion loaded with docetaxel for lung cancer treatment. Drug Deliv Transl Res. 2018;9:543–54.
García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, et al. Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomaterials (Basel). 2019;9:E638. https://doi.org/10.3390/nano.9040638.
Mishra D, Mishra PK, Dabadghao S, Dubey V, Nahar M, Jain NK. Comparative evaluation of hepatitis B surface antigen-loaded elastic liposomes and ethosomes for human dendritic cell uptake and immune response. Nanomedicine. 2010;6:110–8. https://doi.org/10.1016/jnano200904003.
Zanganeh S, Xu Y, Hamby CV, Backer MV, Backer JM, Zhu Q. Enhanced fluorescence diffuse optical tomography with indocyanine green-encapsulating liposomes targeted to receptors for vascular endothelial growth factor in tumor vasculature. J Biomed Opt. 2013;18:126014. https://doi.org/10.1117/1JBO1812126014.
Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol. 2011;137:1337–42. https://doi.org/10.1007/s00432-011-1003-3.
Ektate K, Munteanu MC, Ashar H, Malayer J, Ranjan A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci Rep. 2018;8:13062. https://doi.org/10.1038/s41598-018-30106-4.
Bartheldyova E, Effenberg R, Masek J, et al. Hyaluronic acid surface modified liposomes prepared via orthogonal aminoxy coupling: synthesis of nontoxic aminoxylipids based on symmetrically alpha-branched fatty acids, preparation of liposomes by microfluidic mixing, and targeting to cancer cells expressing CD44. Bioconjug Chem. 2018;29:2343–56. https://doi.org/10.1021/acsbioconjchem8b00311.
Zhang JL, Srivastava RS, Misra RD. Core-shell magnetite NPs surface encapsulated with smart stimuli-responsive polymer: synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir. 2007;23:6342–51.
Gogoi M, Kumar N, Patra, S In Nanoarchitectonics for smart delivery and -drug targeting (Eds A M Holban, A M Grumezescu,), William Andrew Publishing: Norwich, 2016, 743–782, ISBN 978-0-323-47347-7.
Kunjachan S, Ehling J, Storm G, Kiessling F, Lammers T. Noninvasive imaging of nanomedicines and nanotheranostics: principles, Progress, and Prospects. Chem Rev. 2015;115:10907–37.
Cheng L, Huang FZ, Cheng LF, et al. GE11-modified liposomes for non-small cell lung cancer targeting: preparation, ex vitro and in vivo evaluation. Int J Nanomedicine. 2014;9:921–35.
Lin C, Zhang X, Chen H, et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018;25:256–66.
Song XL, Ju RJ, Xiao Y, et al. Application of multifunctional targeting epirubicin liposomes in the treatment of non-small-cell lung cancer. Int J Nanomedicine. 2017;12:7433–51.
Ganesan P, Ramalingam P, Karthivashan G, Ko YT, Choi DK. Recent developments in solid lipid NP and surface-modified solid lipid NP delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int J Nanomedicine. 2018;13:1569–83. https://doi.org/10.2147/IJN.S155593.
Pindiprolu SKSS, Chintamaneni PK, Krishnamurthy PT, Ratna Sree Ganapathineedi K. Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev Ind Pharm. 2018;45:304–13.
Eskiler GG. Synthetically lethal BMN 673 (Talazoparib) loaded solid lipid nanoparticles for BRCA1 mutant triple negative breast cancer. Pharm Res. 2018;35:218.
Oliveira RR, Carrião MS, Pacheco MT, et al. Triggered release of paclitaxel from magnetic solid lipid nanoparticles by magnetic hyperthermia. Mater Sci Eng C. 2018;92:547–53.
Wang W, Zhang L, Chen T, et al. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules. 2017;22:1814.
Chirio D, Peira E, Battaglia L, et al. Lipophilic prodrug of floxuridine loaded into solid lipid nanoparticles: in vitro cytotoxicity studies on different human cancer cell lines. Nanosci Nanotechnol. 2018;18:556–63.
Rosière R, Van Woensel M, Gelbcke M, et al. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol Pharm. 2018;15:899–910.
Fahmy UA. Augmentation of Fluvastatin cytotoxicity against prostate carcinoma PC3 cell line utilizing alpha lipoic–ellagic acid nanostructured lipid carrier formula. AAPS PharmSciTech. 2018;19:3454–61.
Haron AS, Syed Alwi SS, Saiful Yazan L, et al. Cytotoxic effect of thymoquinone-loaded nanostructured lipid carrier (TQ-NLC) on liver cancer cell integrated with hepatitis B genome, Hep3B. Evid Based Complement Alternat Med. 2018;2018:1549805.
Li J, Jin S, Dong XR, Han XF, Wang MY. Construction of artesunate nanoparticles modified by hyaluronic acid and cell-penetrating peptides and its inhibitory effect on cancer cells in vitro. China J Chin Mater Med. 2018;43:3668–75.
Nahak P, Gajbhiye RL, Karmakar G, et al. Orcinol glucoside loaded polymer-lipid hybrid nanostructured lipid carriers: potential cytotoxic agents against gastric Colon and Hepatoma Carcinoma Cell Lines. Pharm Res. 2018;35:198.
Wei Q, Yang Q, Wang Q, et al. Formulation, characterization, and pharmacokinetic studies of 6-gingerol-loaded nanostructured lipid carriers. AAPS Pharm Sci Tech. 2018;19:3661–9.
Ghaffari M, Dehghan G, Abedi-Gaballu F, et al. Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur J Pharm Sci. 2018;122:311–30. https://doi.org/10.1016/jejps201807020.
Shadrack DM, Swai HS, Munissi JJE, Mubofu EB, Nyandoro SS. Polyamidoamine dendrimers for enhanced solubility of small molecules and other desirable properties for site specific delivery: insights from experimental and computational studies. Molecules. 2018;23:E1419. https://doi.org/10.3390/molecules23061419.
Liu J, Liu J, Chu L, et al. Novel peptide-dendrimer conjugates as drug carriers for targeting nonsmall cell lung cancer, conjugates as drug carriers for targeting nonsmall cell lung cancer. Int J Nanomedicine. 2010;6:59–69.
Ryan GM, Kaminskas LM, Kelly BD, et al. Pulmonary administration of PEGylated polylysine dendrimers: absorption from the lung versus retention within the lung is highly size-dependent. Mol Pharm. 2013;10:2986–95.
Kaminskas LM, McLeod VM, Ryan GM, et al. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J Control Release. 2014;183:18–26.
Hasanzadeh M, Babaie P, Jouyban-Gharamaleki V, Jouyban A. The use of chitosan as a bioactive polysaccharide in non-invasive detection of malondialdehyde biomarker in human exhaled breath condensate: a new platform towards diagnosis of some lung disease. Int J Biol Macromol. 2018;120(Pt B):2482–92. https://doi.org/10.1016/jijbiomac.2018.09.018.
Shirvalilou S, Khoei S, Khoee S, Raoufi NJ, Karimi MR, Shakeri-Zadeh A. Development of a magnetic nano-graphene oxide Carrier for improved glioma-targeted drug delivery and imaging: In vitro and in vivo evaluations. Chem Biol Interact. 2018;S0009–2797:30160–1. https://doi.org/10.1016/jcbi.2018.08.027.
Niikura K, Matsunaga T, Suzuki T, et al. Gold NPs as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–38. https://doi.org/10.1021/nn3057005.
Kim YH, Min KH, Wang Z, et al. Development of sialic acid-coated NPs for targeting cancer and efficient evasion of the immune system. Theranostics. 2017;7:962–73. https://doi.org/10.7150/thno.19061.
Peng G, Tisch U, Adams O, et al. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat Nanotechnol. 2009;4:669–73.
Ramalingam V, Varunkumar K, Ravikumar V, Rajaram R. Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer. Sci Rep. 2018;8:3815.
Kim SW, Lee YK, Lee JY, Hong JH, Khang D. PEGylated anticancer-carbon nanotubes complex targeting mitochondria of lung cancer cells. Nanotechnology. 2017;28:465102.
Singh RP, Sharma G, Singh S, et al. Chitosan-folate decorated carbon nanotubes for site specific lung cancer delivery. Mater Sci Eng C. 2017;77:446–58.
Subramanian A, Manigandan A, Sivashankari PR, Sethuraman S. Development of nanotheranostics against metastatic breast cancer--a focus on the biology & mechanistic approaches. Biotechnol Adv. 2015;33:1897–911. https://doi.org/10.1016/jbiotechadv.2015.10.002.
Akhter MH, Rizwanullah M, Ahmad J, Ahsan MJ, Mujtaba MA, Amin S. Nanocarriers in advanced drug targeting: setting novel paradigm in cancer therapeutics. Artif Cells Nanomed Biotechnol. 2018;46:873–84. https://doi.org/10.1080/2169140120171366333.
Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–46. https://doi.org/10.1016/jjconrel201008027.
Chen X, Wang Q, Liu L. Double-sided effect of tumor microenvironment on platelets targeting NPs. Biomaterials. 2018;183:258–67. https://doi.org/10.1016/jbiomaterials201807005.
Gao Y, Xie J, Chen H, et al. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol Adv. 2014;32:761–77. https://doi.org/10.1016/jbiotechadv201310013.
Hussain Z, Khan S, Imran M, Sohail M, Shah SWA, de Matas M. PEGylation: a promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res. 2019;9:721–34. https://doi.org/10.1007/s13346-019-00631-4.
Freichels H, Pourcelle V, Auzely-Velty R, Marchand-Brynaert J, Jerome C. Synthesis of poly(lactide-co-glycolide-co-epsilon-caprolactone)-graft-mannosylated poly(ethylene oxide) copolymers by combination of “clip” and “click” chemistries. Biomacromolecules. 2012;13:760–8. https://doi.org/10.1021/bm201690w.
Wesch D, Peters C, Oberg HH, Pietschmann K, Kabelitz D. Modulation of gammadelta T-cell responses by TLR ligands. Cell Mol Life Sci. 2011;68:2357–70. https://doi.org/10.1007/s00018-011-0699-1.
Silva JM, Videira M, Gaspar R, Preat V, Florindo HF. Immune system targeting by biodegradable NPs for cancer vaccines. J Control Release. 2013;168:179–99. https://doi.org/10.1016/jjconrel201303010.
van Kooyk Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem Soc Trans. 2008;36:1478–81. https://doi.org/10.1042/BST0361478.
Unger WW, van Kooyk Y. Dressed for success’ C-type lectin receptors for the delivery of glyco-vaccines to dendritic cells. Curr Opin Immunol. 2011;23:131–7. https://doi.org/10.1016/jcoi2010.11011.
Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62:949–54. https://doi.org/10.1007/s00262-013-1427-5.
Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–235. https://doi.org/10.1039/c2cs35265f.
Bietenbeck M, Florian A, Faber C, Sechtem U, Yilmaz A. PEGylation strategies for active targeting of PLA/PLGA NPs. J Biomed Mater Res A. 2009;91:263–76. https://doi.org/10.1002/jbma32247.
Tatiparti K, Sau S, Gawde KA, et al. Copper-free ‘click’ chemistry-based synthesis and characterization of carbonic anhydrase-IX anchored albumin-paclitaxel NPs for targeting tumor hypoxia. Int J Mol Sci. 2018;19:E838. https://doi.org/10.3390/ijms19030838.
Bhargava A, Bunkar N, Khare NK, Mishra D, Mishra PK. Nanoengineered strategies to optimize dendritic cells for gastrointestinal tumor immunotherapy: from biology to translational medicine. Nanomedicine (London). 2014;9:2187–202. https://doi.org/10.2217/nn.14.115.
Yan L, Crayton SH, Thawani JP, Amirshaghaghi A, Tsourkas A, Cheng Z. A pH-responsive drug-delivery platform based on glycol chitosan-coated liposomes. Small. 2015;11:4870–4. https://doi.org/10.1002/smll201501412.
Kale AA, Torchilin VP. Environment-responsive multifunctional liposomes. Methods Mol Biol. 2010;605:213–42. https://doi.org/10.1007/978-1-60327-360-2_15.
Yu MK, Park J, Jon S. Magnetic nanoparticles and their applications in image-guided drug delivery. Drug Deliv Transl Res. 2012;2:3–21. https://doi.org/10.1007/s13346-011-0049-8.
Stephan SB, Taber AM, Jileaeva I, et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat Biotechnol. 2015;33:97–101. https://doi.org/10.1038/nbt3104.
Abedin MR, Umapathi S, Mahendrakar H, et al. Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications. J Nanobiotechnology. 2018;16:80. https://doi.org/10.1186/s12951-018-0405-7.
Jauregui R, Srinivasan S, Vojtech LN, et al. Temperature-responsive magnetic NPs for enabling affinity separation of extracellular vesicles. ACS Appl Mater Interfaces. 2018;10:33847–56. https://doi.org/10.1021/acsami8b09751.
Wabler M, Zhu W, Hedayati M, et al. Magnetic resonance imaging contrast of iron oxide NPs developed for hyperthermia is dominated by iron content. Int J Hyperthermia. 2014;30:192–200. https://doi.org/10.3109/02656736.2014.913321.
Unterweger H, Dezsi L, Matuszak J, et al. Dextran-coated superparamagnetic iron oxide NPs for magnetic resonance imaging: evaluation of size-dependent imaging properties, storage stability and safety. Int J Nanomedicine. 2018;13:1899–915. https://doi.org/10.2147/IJN.S156528.
Yang QQ, Wei XL, Fang YP, et al. Nanochemoprevention with therapeutic benefits: an updated review focused on epigallocatechin gallate delivery. Crit Rev Food Sci Nutr. 2019;23:1–22. https://doi.org/10.1080/10408398.2019.1565490.
Ferguson PM, Slocombe A, Tilley RD, Hermans IF. Using magnetic resonance imaging to evaluate dendritic cell-based vaccination. PLoS One. 2013;8:e65318. https://doi.org/10.1371/journalpone0065318.
Gholipourmalekabadi M, Mobaraki M, Ghaffari M, et al. Targeted drug delivery based on gold np derivatives. Curr Pharm Des. 2017;23:2918–29. https://doi.org/10.2174/1381612823666170419105413.
Wallace A, Kapoor V, Sun J, et al. Transforming growth factor-β receptor blockade augments the effectiveness of adoptive T cell therapy of established solid cancers. Clin Cancer Res. 2008;14:3966–74.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–96. https://doi.org/10.1038/s41573-018-0006-z.
Stephan MT, Moon JJ, Um SH, Bersthteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–41.
Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–87.
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human. Cancer Sci. 2015;348:62–8.
Smith T, Stephan SB, Moffett HF, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–20.
Smith TT, Moffett HF, Stephan SB, et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest. 2017;127:2176–91.
Yang S, Wen J, Li H, et al. Aptamer-engineered natural killer cells for cell-specific adaptive immunotherapy. Small. 2019;15:e1900903. https://doi.org/10.1002/smll201900903.
Meyer RA, Sunshine JC, Perica K, et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–25.
Kosmides AK, Meyer RA, Hickey JW, et al. Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials. 2017;118:16–26.
Sakhtianchi R, Minchin RF, Lee KB, Alkilany AM, Serpooshan V, Mahmoudi M. Exocytosis of nanoparticles from cells: role in cellular retention and toxicity. Adv Colloid Interface Sci. 2013;201-202:18–29. https://doi.org/10.1016/jcis201310013.
Zaki NM, Nasti A, Tirelli N. Nanocarriers for cytoplasmic delivery: cellular uptake and intracellular fate of chitosan and hyaluronic acid-coated chitosan nanoparticles in a phagocytic cell model. Macromol Biosci. 2011;11:1747–60. https://doi.org/10.1002/mabi201100156.
Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–22. https://doi.org/10.1016/jijpharm200503035.
Mottram PL, Leong D, Crimeen-Irwin B, et al. Type 1 and 2 immunity following vaccination is influenced by NP size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4:73–84. https://doi.org/10.1021/mp060096p.
Rice-Ficht AC, Arenas-Gamboa AM, Kahl-McDonagh MM, Ficht TA. Polymeric particles in vaccine delivery. Curr Opin Microbiol. 2010;13:106–12. https://doi.org/10.1016/jmib200912001.
Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–8. https://doi.org/10.1073/pnas0801763105.
Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. NPs target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–13. https://doi.org/10.1002/eji200737984.
Kourtis IC, Hirosue S, de Titta A, et al. Peripherally administered NPs target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS One. 2013;8:e61646. https://doi.org/10.1371/journalpone0061646.
Singh D, McMillan JM, Liu XM, et al. Formulation design facilitates magnetic NP delivery to diseased cells and tissues. Nanomedicine (London). 2014;9:469–85. https://doi.org/10.2217/nnm.14.4.
Jahan ST, Sadat SMA, Walliser M, Haddadi A. Targeted therapeutic nps: an immense promise to fight against cancer. J Drug Deliv 2017; 9090325. https://doi.org/10.1155/2017/9090325.
Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic NPs. Philos Trans A Math Phys Eng Sci. 2010;368:1333–83. https://doi.org/10.1098/rsta20090273.
Mohanan D, Slutter B, Henriksen-Lacey M, et al. Administration routes affect the quality of immune responses: a cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–9. https://doi.org/10.1016/jjconrel201008012.
Maji M, Mazumder S, Bhattacharya S, et al. A lipid based antigen delivery system efficiently facilitates mhc class-i antigen presentation in dendritic cells to stimulate CD8(+) T-cells. Sci Rep. 2016;6:27206. https://doi.org/10.1038/srep27206.
Chandrasekaran S, McGuire MJ, King MR. Sweeping lymph node micrometastases off their feet: an engineered model to evaluate natural killer cell mediated therapeutic intervention of circulating TCs that disseminate to the lymph nodes. Lab Chip. 2014;14:118–27. https://doi.org/10.1039/c3lc50584g.
Acknowledgments
The authors are thankful to the Department of Science & Technology (DST) Government of India, New Delhi, and Indian Council of Medical Research (ICMR) Government of India, New Delhi, for providing necessary financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhargava, A., Mishra, D.K., Tiwari, R. et al. Immune cell engineering: opportunities in lung cancer therapeutics. Drug Deliv. and Transl. Res. 10, 1203–1227 (2020). https://doi.org/10.1007/s13346-020-00719-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00719-2