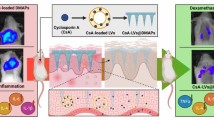
Abstract
The primary aim of the present study was to develop lanolin-based organogel with enhanced delivery potential and reduced skin irritation for the treatment of hyperkeratotic lesions and scaling. The drug was encapsulated in the lipidic bilayers of organogel. The values of particle size, polydispersity index (PDI), and zeta potential of the developed carrier system was found to be 257.5 nm, 0.272, and −24.9 mV, respectively. The system was pseudoplastic in nature with the yield value of 2.3078 Pa. The skin permeation studies exhibited superiority of the prepared lanolin-based organogel formulation over the conventional gel formulation (CGF). Further, the dermatokinetic studies also confirmed better permeation and enhanced skin bioavailability of SA to epidermis as well as dermis vis-à-vis the CGF. In conclusion, the developed organogel system not only improved the delivery profile of SA but also reduced the skin irritant potential. The current findings can provide a suitable alternative for the development of an effective topical formulation of SA for the treatment of hyperkeratotic lesions.












Similar content being viewed by others
References
Dougados M. Why and how to use NSAIDs in osteoarthritis. J Cardiovasc Pharmacol. 2006;47:49–54.
Rubin L. Hyperkeratosis in response to mechanical irritation. J Investig Dermatol. 1949;13:313–5.
Baden HP, Alper JC. A keratolytic gel containing salicylic acid in propylene glycol. J Invest Dermatol. 1973;61:330–3.
Bikowski J. Hyperkeratosis of the heels: treatment with salicylic acid in a novel delivery system. Skinmed. 2004;3:350–1.
Nordström FL, Rasmuson ÅC. Solubility and melting properties of salicylic acid. J Chem Eng Data. 2006;51:1668–71.
Simmonite N, Lang L, West S, Day S. Salicylic acid in the treatment of corns. Foot. 1994;4:145–50.
Nour SA, Badawi AA, Sakran WS, El-Mancy SMS. Preparation and evaluation of microemulsion systems containing salicylic acid. AAPS PharmSciTech. 2009;10:1081–4.
Moore DJ, Mateus R, Hadgraft J, Lane ME. Percutaneous absorption of salicylic acid—in vitro and in vivo studies. Int J Pharm. 2014;475:471–4.
Gupta M, Agrawal U, Vyas SP. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin Drug Deliv. 2012;9:783–804.
Pandey M, Belgamwar V, Gattani S, Surana S, Tekade A. Pluronic lecithin organogel as a topical drug delivery system. Drug Deliv. 2010;17:38–47.
Kumar R, Katare OP. Lecithin organogels as a potential phospholipid-structured system for topical drug delivery: a review. AAPS PharmSciTech. 2005;6:E298–310.
Vintiloiu A, Leroux JC. Organogels and their use in drug delivery—a review. J Control Release. 2008;125:179–92.
Murdan S. Organogels in drug delivery. Expert opinion on drug delivery. 2005;2:489–505.
Sagiri SS, Behera B, Pal K, Basak P. Lanolin-based organogels as a matrix for topical drug delivery. J Appl Polym Sci. 2013;128:3831–9.
Sahoo S, Kumar N, Bhattacharya C, Sagiri S, Jain K, Pal K, et al. Organogels: properties and applications in drug delivery. Designed monomers and polymers. 2011;14:95–108.
Katare OP, Kumar R. Lecithin organogels as a potential phospholipid-structured system for topical drug delivery: a review. AAPS PharmSciTech. 2005;6:E298–310.
Singh N, Khullar N, Kakkar V, Kaur IP. Sesamol loaded solid lipid nanoparticles: a promising intervention for control of carbon tetrachloride induced hepatotoxicity. BMC Complement Altern Med. 2015;15:142.
Raza K, Singh B, Mahajan A, Negi P, Bhatia A, Katare OP. Design and evaluation of flexible membrane vesicles (FMVs) for enhanced topical delivery of capsaicin. J Drug Target. 2011;19:293–302.
Bhatia A, Singh B, Raza K, Wadhwa S, Katare OP. Tamoxifen-loaded lecithin organogel (LO) for topical application: development, optimization and characterization. Int J Pharm. 2013;444:47–59.
Chhibber T, Wadhwa S, Chadha P, Sharma G, Katare OP. Phospholipid structured microemulsion as effective carrier system with potential in methicillin sensitive Staphylococcus aureus (MSSA) involved burn wound infection. J Drug Target. 2015;1-10
Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258:141–51.
Yap KL, Liu X, Thenmozhiyal JC, Ho PC. Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexes by phase solubility, photostability, physicochemical and computational analysis. Eur J Pharm Sci. 2005;25:49–56.
Upadhyay KK, Tiwari C, Khopade AJ, Bohidar HB, Jain SK. Sorbitan ester organogels for transdermal delivery of sumatriptan. Drug Dev Ind Pharm. 2007;33:617–25.
Bhatia A, Raza K, Singh B, Katare OP. Phospholipid-based formulation with improved attributes of coal tar. J Cosmet Dermatol. 2009;8:282–8.
Patel J, Garala K, Basu B, Raval M, Dharamsi A. Solubility of aceclofenac in polyamidoamine dendrimer solutions. Int J Pharma Investig. 2011;1(3):135–8.
Basu S, Shivhare US, Raghavan GSV. Time dependent rheological characteristics of pineapple jam. Int J Food Eng. 2007;3(3):1–10.
Agarwal R, Katare OP, Vyas SP. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 2001;228:43–52.
Sharma G, Dhankar G, Thakur K, Raza K, Katare OP. Benzyl benzoate-loaded microemulsion for topical applications: enhanced dermatokinetic profile and better delivery promises. AAPS PharmSciTech. 2016;17:1221–31.
Guth K, Schafer-Korting M, Fabian E, Landsiedel R, van Ravenzwaay B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol in Vitro. 2015;29:113–23.
Sharma G, Kaur M, Raza K, Thakura K, Katare O. Aceclofenac–β-cyclodextrin-vesicles: a dual carrier approach for skin with enhanced stability, efficacy and dermatokinetic profile. RSC Adv. 2016;6:20713–27.
N'Dri-Stempfer B, Navidi WC, Guy RH, Bunge AL. Improved bioequivalence assessment of topical dermatological drug products using dermatopharmacokinetics. Pharm Res. 2009;26:316–28.
Pershing LK, Nelson JL, Corlett JL, Shrivastava SP, Hare DB, Shah VP. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740–51.
Raza K, Katare OP, Setia A, Bhatia A, Singh B. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nano-emulsomes. J Microencapsul. 2013;30:225–36.
Negi P, Singh B, Sharma G, Katare OP. Enhanced topical delivery of lidocaine via ethosomes-based hydrogel: ex-vivo and In-vivo evaluation. J Nanopharm Drug Deliv. 2014;2:1–10.
Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:702–5.
Negi P, Singh B, Sharma G, Beg S, Katare OP. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J Microencapsul. 2015;32:419–31.
ICH, Q1A(R2). Stability testing of new drug substances and products, In: International conference on harmonisation, IFPMA, Geneva, 2003.
Raza K, Shareef MA, Singal P, Sharma G, Negi P, Katare OP. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. J Liposome Res. 2014;24:290–6.
Raza K, Singh B, Lohan S, Sharma G, Negi P, Yachha Y, et al. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int J Pharm. 2013;456:65–72.
Yoo JK, Choo YK, Kwak DH, Lee JM, Lim CY, Lee JH, et al. Protective effects of agonistic anti-4-1BB antibody on the development of imiquimod-induced psoriasis-like dermatitis in mice. Immunol Lett. 2016;178:131–9.
Christensen TE, Callis KP, Papenfuss J, Hoffman MS, Hansen CB, Wong B, et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397–403.
Amarji B, Garg NK, Singh B, Katare OP. Microemulsions mediated effective delivery of methotrexate hydrogel: more than a tour de force in psoriasis therapeutics. J Drug Target. 2016;24:147–60.
Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, et al. Mouse model of imiquimod-induced psoriatic itch. Pain. 2016;157:2536–43.
Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380:738–46. doi:10.1016/S0140-6736(12)60642-4. Epub 2012 Jun 29
van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–45.
Pal K, Banthia A, Majumdar D. Polyvinyl alcohol—gelatin patches of salicylic acid: preparation, characterization and drug release studies. J Biomater Appl. 2006;21:75–91.
Kostarelos K, Luckham PF, Tadros TF. Steric stabilisation of phospholipid vesicles by block copolymers: vesicle flocculation and osmotic swelling caused by monovalent and divalent cations. J Chem Soc Faraday Tran. 1998;94:91–145.
Jatav MP, Ramteke S. Formulation and evaluation of lecithin organogel for treatment of arthritis. International Journal of Scientific World. 2015;3:267–74.
Doukas AG, Soukos NS, Babusis S, Appa Y, Kollias N. Fluorescence excitation spectroscopy for the measurement of epidermal proliferation. Photochem Photobiol. 2001;74:96–102.
Ahmed K, Gribbon PN, Jones MN. The application of confocal microscopy to the study of liposome adsorption onto bacterial biofilms. J Liposome Res. 2002;12:285–300.
Bhalerao S, Raje HA. Preparation, optimization, characterization, and stability studies of salicylic acid liposomes. Drug Dev Ind Pharm. 2003;29:451–67.
Hou D, Xie C, Huang K, Zhu C. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials. 2003;24:1781–5.
Nasr M, Mansour S, Mortada ND, El Shamy AA. Lipospheres as carriers for topical delivery of aceclofenac: preparation, characterization and in vivo evaluation. AAPS PharmSciTech. 2008;9:154–62.
Tunesi S, Anderson M. Influence of chemisorption on the photodecomposition of salicylic acid and related compounds using suspended titania ceramic membranes. J Phys Chem. 1991;95:3399–405.
Jagdale SC, Khawale PS, Kuchekar BS, Chabukswar AR. Development and evaluation of pluronic lecithin organogel topical delivery of tapentadol. American Journal of Pharmaceutical Sciences and Nanotechnology. 2015;2:1–21.
Kantaria S, Rees GD, Lawrence MJ. Gelatin-stabilised microemulsion-based organogels: rheology and application in iontophoretic transdermal drug delivery. J Control Release. 1999;60:355–65.
Balaguru S, Ramya Devi D, Vedha Hari BN. Organogel: an ideal drug delivery carrier for nonsteroidal anti-inflammatory drugs through topical Route. Int J Pharm Qual Assur. 2015;6:32–7.
Hurler J, Engesland A, Poorahmary Kermany B, Škalko-Basnet N. Improved texture analysis for hydrogel characterization: gel cohesiveness, adhesiveness, and hardness. J Appl Polym Sci. 2012;125:180–8.
Bhowmick M, Sengodan T. Mechanisms, kinetics and mathematical modelling of transdermal permeation-an updated review. Int J Res Dev Pharm L Sci. 2013;2:636–41.
Leveque N, Makki S, Hadgraft J, Humbert P. Comparison of Franz cells and microdialysis for assessing salicylic acid penetration through human skin. Int J Pharm. 2004;269:323–8.
Sharma G, Kamboj S, Thakur K, Negi P, Raza K, Katare OP. Delivery of thermoresponsive-tailored mixed micellar nanogel of lidocaine and prilocaine with improved dermatokinetic profile and therapeutic efficacy in topical anaesthesia. AAPS PharmSciTech. 2016; doi:10.1208/s12249-016-0561-8.
Dreher F, Walde P, Luisi P, Elsner P. Human skin irritation studies of a lecithin microemulsion gel and of lecithin liposomes. Skin Pharmacol Physiol. 1996;9:124–9.
Imayama S, Ueda S, Isoda M. Histologic changes in the skin of hairless mice following peeling with salicylic acid. Arch Dermatol. 2000;136:1390–5.
Acknowledgements
Authors are thankful to University Grants Commission (UGC), New Delhi, India, for research grant, Psyco Remedies Limited, Ludhiana, India, and M/s Phospholipid GmbH, Nattermannallee, Germany, for the ex-gratis supply of drug and phospholipids.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, G., Devi, N., Thakur, K. et al. Lanolin-based organogel of salicylic acid: evidences of better dermatokinetic profile in imiquimod-induced keratolytic therapy in BALB/c mice model. Drug Deliv. and Transl. Res. 8, 398–413 (2018). https://doi.org/10.1007/s13346-017-0364-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0364-9