Abstract
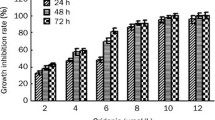
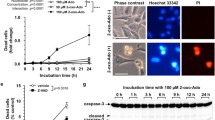
6-(2-hydroxybenzylamino)-9-D-ribofuranosylpurine (ortho-topolin riboside, oTR), a naturally occurring cytokinin and nucleoside analog has potential anticancer effects. However, the molecular mechanisms remain elusive. We found that oTR strongly inhibited Acute myeloid leukemia HL-60 cell proliferation, altered the cell cycle, induced cytochrome c release from mitochondria into the cytosol, and increased caspase-3 activity. Apoptosis was confirmed by DNA ladder formation following gel electrophoresis. These results indicated that oTR induced apoptosis through activation of the intrinsic mitochondrial pathway. Moreover, the apoptosis was significantly suppressed by the adenosine transporter inhibitor dipyridamole and adenosine kinase inhibitor A-134974. These data indicated that cellular uptake of oTR was an active process involving an adenosine transporter, and subsequently phosphorylated by an adenosine kinase. Taken together, Our study suggests that oTR is taken up by HL-60 cells, converted to the phosphorylated form, and induces apoptosis.
Similar content being viewed by others
References
Santini, V. et al. Butyrate-stable monosaccharide derivatives induce maturation and apoptosis in human acute myeloid leukaemia cells. Br J Haematol 101:529–538 (1998).
Ewald, B., Sampath, D. & Plunkett, W. Nucleoside analogs: Molecular mechanisms signaling cell death. Oncogene 27:6522–6537 (2008).
Jordheim, L. P., Durantel, D., Zoulim, F. & Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov 12:447–464 (2013).
Honma, Y. Control of differentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant redifferentiation-inducing hormones. Drug Develop Res 56:560–560 (2002).
Voller, J. et al. Anticancer activity of natural cytokinins: A structure-activity relationship study. Phytochemistry 71:1350–1359 (2010).
Berge, U., Kristensen, P. & Rattan, S. I. S. Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann N Y Acad Sci 1067:332–336 (2006).
Ishii, Y., Sakai, S. & Honma, Y. Cytokinin-induced differentiation of human myeloid leukemia hl-60 cells is associated with the formation of nucleotides, but not with incorporation into DNA or RNA. Biochim Biophys Acta 1643:11–24 (2003).
Mlejnek, P. Caspase inhibition and n6-benzyladenosine-induced apoptosis in hl-60 cells. J Cell Biochem 83:678–689 (2001).
Choi, B. H. et al. Kinetin riboside preferentially induces apoptosis by modulating bcl-2 family proteins and caspase-3 in cancer cells. Cancer Lett 261:37–45 (2008).
Cabello, C. M. et al. The experimental chemotherapeutic n6-furfuryladenosine (kinetin-riboside) induces rapid atp depletion, genotoxic stress, and cdkn1a(p21) upregulation in human cancer cell lines. Biochem Pharmacol 77:1125–1138 (2009).
Griffaut, B., Bos, R., Maurizis, J. C., Madelmont, J. C. & Ledoigt, G. Cytotoxic effects of kinetin riboside on mouse, human and plant tumour cells. Int J Biol Macromol 34:271–275 (2004).
Laezza, C. et al. N6-isopentenyladenosine inhibits cell proliferation and induces apoptosis in a human colon cancer cell line dld1. Int J Cancer 124:1322–1329 (2009).
Meisel, H., Gunther, S., Martin, D. & Schlimme, E. Apoptosis induced by modified ribonucleosides in human cell culture systems. FEBS Lett 433:265–268 (1998).
Spinola, M., Colombo, F., Falvella, F. S. & Dragani, T. A. N6-isopentenyladenosine: A potential therapeutic agent for a variety of epithelial cancers. Int J Cancer 120:2744–2748 (2007).
Laezza, C. et al. N6-isopentenyladenosine arrests tumor cell proliferation by inhibiting farnesyl diphosphate synthase and protein prenylation. FASEB J 20:412–418 (2006).
Tiedemann, R. E. et al. Identification of kinetin riboside as a repressor of ccnd1 and ccnd2 with preclinical antimyeloma activity. J Clin Invest 118:1750–1764 (2008).
Mittelman, A., Evans, J. T. & Chheda, G. B. Cytokinins as chemotherapeutic-agents. Ann Ny Acad Sci 255:225–234 (1975).
Hewett, E. W. & Wareing, P. F. Cytokinins in populus x robusta schneid -complex in leaves. Planta 112:225–233 (1973).
Cano-Soldado, P. & Pastor-Anglada, M. Transporters that translocate nucleosides and structural similar drugs: Structural requirements for substrate recognition. Med Res Rev 32:428–457 (2012).
Prathapan, A., Vineetha, V. P. & Raghu, K. G. Protective effect of boerhaavia diffusa l. Against mitochondrial dysfunction in angiotensin ii induced hypertrophy in h9c2 cardiomyoblast cells. Plos One 9 (2014).
Minuesa, G. et al. Drug uptake transporters in antiretroviral therapy. Pharmacol Ther 132:268–279 (2011).
Mok, M. C., Martin, R. C. & Mok, D. W. S. Cytokinins: Biosynthesis, metabolism and perception. In Vitro Cell Dev-Pl 36:102–107 (2000).
Javadov, S. et al. Antihypertrophic effect of na+/h+ exchanger isoform 1 inhibition is mediated by reduced mitogen-activated protein kinase activation secondary to improved mitochondrial integrity and decreased generation of mitochondrial-derived reactive oxygen species. J Pharmacol Exp Ther 317:1036–1043 (2006).
Javadov, S. & Karmazyn, M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem 20:1–22 (2007).
Javadov, S., Karmazyn, M. & Escobales, N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharm Exp Ther 330:670–678 (2009).
Matsumoto, S., Friberg, H., Ferrand-Drake, M. & Wieloch, T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J Cerebr Blood F Met 19:736–741 (1999).
Hu, W. & Kavanagh, J. J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 4:721–729 (2003).
Spierings, D. et al. Connected to death: The (unexpurgated) mitochondrial pathway of apoptosis. Science 310:66–67 (2005).
Liu, X. et al. Molecular basis for g2 arrest induced by 2’-c-cyano-2’-deoxy-1-beta-d-arabino-pentofuranosylcytosine and consequences of checkpoint abrogation. Cancer Res 65:6874–6881 (2005).
Chen, Y. H. & Sanchez, Y. Chk1 in the DNA damage response: Conserved roles from yeasts to mammals. DNA Repair 3:1025–1032 (2004).
Shi, Z. et al. S-phase arrest by nucleoside analogues and abrogation of survival without cell cycle progression by 7-hydroxystaurosporine. Cancer Res 61:1065–1072 (2001).
Wang, J., Li, X. R., Liu, Y. B. & Zhao, X. Salt stress induces programmed cell death in thellungiella halophila suspension-cultured cells. J Plant Physiol 167:1145–1151 (2010).
Wang, L., Sun, C., Wang, Z. H. & Guo, G. Q. Mechanism of apoptotosis induced by ortho-topolin riboside in human hepatoma cell line smmc-7721. Food Chem Toxicol 50:1962–1968 (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Yu, D.L., Zhang, H.W. et al. Ortho-topolin riboside induces apoptosis in Acute myeloid leukemia HL-60 cells. Mol. Cell. Toxicol. 12, 159–166 (2016). https://doi.org/10.1007/s13273-016-0020-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-016-0020-3