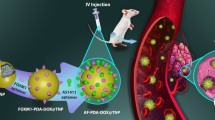
Abstract
Nanocarriers with pH-sensitive functionality are of great interest in the development of pH-dependent drug release compounds in acidic tumor microenvironments. A new polyelectrolyte block copolymer, poly[(benzyl-L-aspartate)-co-(N-(3-aminopropyl) imidazole-L-aspartamide)]-poly(ethylene glycol) (PABI-PEG), was prepared by one-step modulation to produce pH-sensitive nanocarriers. PABI-PEG formed a stable nanocarrier at pH values above 7.4 and was destabilized in acidic conditions (pH 6.5) through the protonation of the imidazole groups. Docetaxel loaded micelle (DLM) exhibited pH-dependent drug release through structural conversion due to the protonation of the imidazole groups on the PABI block. The critically low micelle concentration of PABI-PEG at physiological pH and the pH-dependent drug release would result to high stability and restrict drug loss during systemic circulation which may lower the toxicity of normal tissue to physiological pH. Additionally, the extracellular tumor pH (<7.0) and early endosomal pH (<6.5) environments triggered the disintegration of micelles, producing higher drug release compared to other normal tissues and blood (pH 7.4). Therefore, PABI-PEG may be a pHsensitive drug delivery method for cancer chemotherapy.

Similar content being viewed by others
References
A. S. Hoffman, Artif. Organs, 19, 458 (1995).
B. Jeong and A. Gutowska, Trends Biotechnol., 20, 305 (2002).
M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov, and S. Minko, Nat. Mater., 9, 101 (2010).
T. Ramasamy, H. B. Ruttala, B. Gupta, B. K. Poudel, H.-G. Choi, C. S. Yong, and J. O. Kim, J. Control. Release, 258, 226 (2017).
E.-K. Lim, B. H. Chung, and S. Chung, Curr. Drug Targets, 19, 300 (2018).
T. Sim, C. Lim, N. H. Hoang, and K. T. Oh, J. Pharm. Investig., 47, 383 (2017).
J. H. Choi, Y. J. Lee, and D. Kim, J. Pharm. Investig., 47, 51 (2017).
Y. H. Choi and H.-K. Han, J. Pharm. Investig., 48, 43 (2018).
X. Ma and R. O. Williams, J. Pharm. Investig., 48, 61 (2018).
Q.-V. Le, J. Choi, and Y.-K. Oh, J. Pharm. Investig., 48, 527 (2018).
T. Sim, C. Lim, N. H. Hoang, H. Joo, J. W. Lee, D.-W. Kim, E. S. Lee, Y. S. Youn, J. O. Kim, and K. T. Oh, J. Pharm. Investig., 46, 351 (2016).
E. Cabane, X. Zhang, K. Langowska, C. G. Palivan, and W. Meier, Biointerphases, 7, 9 (2012).
I. Y. Galaev and B. Mattiasson, Trends Biotechnol., 17, 335 (1999).
A. Kumar, A. Srivastava, I. Y. Galaev, and B. Mattiasson, Prog. Polym. Sci., 32, 1205 (2007).
Y. Osada and J. Gong, Prog. Polym. Sci., 18, 187 (1993).
J. J. Li, F. Zhao, and J. Li, Adv. Biochem. Eng. Biotechnol., 125, 207 (2011).
B. Priya, P. Viness, E. C. Yahya, and C. d. T. Lisa, Biomed. Mater., 4, 022001 (2009).
C. Lim, Y. S. Youn, K. S. Lee, N. H. Hoang, T. Sim, E. S. Lee, and K. T. Oh, Int. J. Nanomed., 11, 703 (2016).
T. Sim, G. Park, H. Min, S. Kang, C. Lim, S. Bae, E. S. Lee, Y. S. Youn, and K. T. Oh, J. Bioact. Compat. Pol., 32, 280 (2016).
H.-T. Song, N. H. Hoang, J. M. Yun, Y. J. Park, E. H. Song, E. S. Lee, Y. S. Youn, and K. T. Oh, Colloid. Surface B, 144, 73 (2016).
C. Lim, T. Sim, N. H. Hoang, and K. T. Oh, Colloid. Surface B, 153, 10 (2017).
E. S. Lee, Z. Gao, D. Kim, K. Park, I. C. Kwon, and Y. H. Bae, J. Control. Release, 129, 228 (2008).
J. H. Kim, Y. T. Oh, K. S. Lee, J. M. Yun, B. T. Park, and K. T. Oh, Macromol. Res., 19, 453 (2011).
E.-S. Lee, J.-H. Kim, J.-M. Yun, K.-S. Lee, G.-Y. Park, B.-J. Lee, and K.-T. Oh, J. Pharm. Investig., 40, 45 (2010).
E. S. Lee, K. Na, and Y. H. Bae, J. Control. Release, 103, 405 (2005).
E. S. Lee, K. Na, and Y. H. Bae, Nano Lett., 5, 325 (2005).
K. T. Oh, H. Yin, E. S. Lee, and Y. H. Bae, J. Mater. Chem., 17, 3987 (2007).
D. Schmaljohann, Adv. Drug Deliv. Rev., 58, 1655 (2006).
L. Tian and Y. H. Bae, Colloid. Surface B, 99, 116 (2012).
J. Kim, Y. Oh, K. Lee, J. Yun, B. Park, and K. Oh, Macromol. Res., 19, 453 (2011).
E. S. Lee, J. H. Kim, T. Sim, Y. S. Youn, B.-J. Lee, Y. T. Oh, and K. T. Oh, J. Mater. Chem. B, 2, 1152 (2014).
T. Sim, C. Lim, N. H. Hoang, J. E. Kim, E. S. Lee, Y. S. Youn, and K. T. Oh, J. Mater. Chem. B, 5, 8498 (2017).
T. Sim, C. Lim, Y. H. Cho, E. S. Lee, Y. S. Youn, and K. T. Oh, J. Appl. Polym. Sci., 135, 46268 (2018).
S. Zalipsky and G. Barany, J. Bioact. Compat. Pol., 5, 227 (1990).
K. Yoon, H. C. Kang, L. Li, H. Cho, M.-K. Park, E. Lee, Y. H. Bae, and K. M. Huh, Polym. Chem-UK, 6, 531 (2015).
M. Nakanishi, J.-S. Park, W.-D. Jang, M. Oba, and K. Kataoka, React. Funct. Polym., 67, 1361 (2007).
E. A. Lysenko, T. K. Bronich, E. V. Slonkina, A. Eisenberg, V. A. Kabanov, and A. V. Kabanov, Macromolecules, 35, 6351 (2002).
J. Aguiar, P. Carpena, J. Molina-Bolivar, and C. C. Ruiz, J, Colloid Interf. Sci., 258, 116 (2003).
K. Kalyanasundaram and J. Thomas, J. Am. Chem. Soc., 99, 2039 (1977).
E. S. Lee, D. Kim, Y. S. Youn, K. T. Oh, and Y. H. Bae, Angew. Chem. Int. Ed. Engl., 47, 2418 (2008).
M. Lee, N. Oh, K. Oh, Y. Youn, and E. Lee, J. Pharm. Investig., 44, 351 (2014).
S. Kooijmans, L. Fliervoet, R. Van Der Meel, M. Fens, H. Heijnen, P. v. B. e. Henegouwen, P. Vader, and R. Schiffelers, J. Control. Release, 224, 77 (2016).
P. L. Turecek, M. J. Bossard, F. Schoetens, and I. A. Ivens, J, Pharm. Sci., 105, 460 (2016).
N. M. Oh, K. T. Oh, Y. S. Youn, D. K. Lee, K. H. Cha, D. H. Lee, and E. S. Lee, Colloid. Surface B, 101, 298 (2013).
S. Pal and S. Moulik, J. Lipid. Res., 24, 1281 (1983).
E. S. Lee, K. Na, and Y. H. Bae, J. Control. Release, 91, 103 (2003).
G. M. Kim, Y. H. Bae, and W. H. Jo, Macromol. Biosci., 5, 1118 (2005).
Y. H. Choi and H.-K. Han, J. Pharm. Investig., 1 (2018).
C.-Y. Su, J.-J. Liu, Y.-S. Ho, Y.-Y. Huang, V. H.-S. Chang, D.-Z. Liu, L.-C. Chen, H.-O. Ho, and M.-T. Sheu, Biopharmaceutics, 123, 9 (2018).
T. Sim, J. E. Kim, N. H. Hoang, J. K. Kang, C. Lim, D. S. Kim, E. S. Lee, Y. S. Youn, H.-G. Choi, and H.-K. Han, Drug Deliv., 25, 1362 (2018).
Y. Shi, M. J. va. Steenbergen, E. A. Teunissen, L. S. Novo, S. Gradmann, M. Baldus, C. F. va. Nostrum, and W. E. J. B. Hennink, Biomacromolecules, 14, 1826 (2013).
C. Lim, J. Moon, T. Sim, N. H. Hoang, W. R. Won, E. S. Lee, Y. S. Youn, H.-G. Choi, K. Oh, and K. T. Oh, Int. J. Nanomed., 13, 4627 (2018).
M. S. Muthu, R. V. Kutty, Z. Luo, J. Xie, and S.-S. Feng, Biomaterials, 39, 234 (2015).
T. Ramasamy, J. Y. Choi, H. J. Cho, S. K. Umadevi, B. S. Shin, H. G. Choi, C. S. Yong, and J. O. Kim, Pharm. Res., 32, 1947 (2015).
C.-Y. Su, J.-J. Liu, Y.-S. Ho, Y.-Y. Huang, V. H.-S. Chang, D.-Z. Liu, L.-C. Chen, H.-O. Ho, and M.-T. Sheu, Eur. J. Pharm. Biopharm., 123, 9 (2018).
O. S. Qureshi, H.-S. Kim, A. Zeb, J.-S. Choi, H.-S. Kim, J.-E. Kwon, M.-S. Kim, J.-H. Kang, C. Ryou, J.-S. Park, and J.-K. Kim, J. Microencapsul., 34, 250 (2017).
Acknowledgment
This research was supported by a grant (16173MFDS542) from the Ministry of Food and Drug Safety in 2019 and by a Chung-Ang University Research Scholarship Grant in 2018.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sim, T., Han, S.M., Lim, C. et al. A pH-Sensitive Polymer for Cancer Targeting Prepared by One-Step Modulation of Functional Side Groups. Macromol. Res. 27, 795–802 (2019). https://doi.org/10.1007/s13233-019-7112-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-019-7112-6