Abstract
Cu2ZnSnS4 (CZTS) is considered to be one of the most promising light absorbing materials for low-cost and high-efficiency thin-film solar cells. It is composed of earth abundant, non-toxic elements. In the present study, wurtzite CZTS nanocone has been synthesized by a green chemistry route. The nanocones have been characterized for its optical, structural and microstructural properties using UV–Vis spectrophotometer, X-ray diffraction, Raman spectroscopy and high-resolution transmission electron microscopy. Optical absorption result shows a band gap of 1.42 eV. XRD and Raman results show wurtzite structure and TEM studies reveal the nanocone structure of the grown material. Growing vertically aligned nanocone structure having smaller diameter shall help in enhancing the light absorption in broader range which shall enhance the efficiency of solar cell. This study is a step in this direction.
Similar content being viewed by others
Introduction
CZTS has a direct band gap of about 1.5 eV which is close to the optimal value for being used as an absorber layer in solar cell. The absorption coefficient of CZTS is greater than 104/cm and therefore only a thin layer (1–2 μm) can absorb most of the incident photons (Zhao and Qiao et al. 2014; Mainz et al. 2014; Fan 2014; Tanaka et al. 2007; Chen et al. 2009; Yu et al. 2012). It is known that nanocone structure is an optimal shape for enhancing the light absorption (Wang and Leu 2012). In the study of Wang et al. it has been reported that nanocone arrays significantly improve the solar absorption and efficiencies over nanowire arrays (Hsu et al. 2008). Through simulations they have shown that nanocones have superior absorption due to reduced reflection from their smaller tip and reduced transmission from their larger base. Breaking the vertical mirror symmetry of nanowires results in a broader absorption spectrum such that overall efficiencies are enhanced (Hsu et al. 2008; Mehta and Kruis 2005). It has also been reported by Mehta et al. that nanocone structure is capable of harvesting photons from the sufficient amount of solar spectrum (Li et al. 2012). The field created at the nanojunction (consisting of nanocone and thin film) will effectively (1) separate electrons and holes when they are generated under illumination, (2) extract electrons from the whole absorber to the small tip-film contact area, (3) increase the velocity of minority carriers when they cross the tip-film junction, minimizing recombination loss at the interface and (4) decrease the number of recombination centers by utilizing smaller contact area. Thus, synthesizing nanocone structure shall be highly useful for enhancing the solar cell efficiency. Considering the above factors, wurtzite-type CZTS nanocone structure has been synthesized in this study by a green technique. These nanocrystals are dispersible in non-polar solvents and film can be fabricated simply by the direct liquid coating method. If these nanocones with smaller dimension be can be deposited vertically on substrate then this shall help in enhancing the efficiency of solar cell. To illustrate the concept; a schematic diagram of CZTS thin-film solar cell having nanocone structure is presented in Fig. 1.
There are several reports on the synthesis of CZTS nanocrystals by hot-injection method (Guo et al. 2010, 2009; Riha et al. 2009). In general, CZTS nanocrystals are mostly in the kesterite or stannite phase (Shavel et al. 2010). It has been reported by Regulacio et al. that in colloidal nanocrystal synthesis, the presence of organic surfactants or capping ligands is known to strongly influence the crystallographic phase, morphology and growth of the nanocrystals (Regulacio et al. 2012). Thus, the formation of metastable phases can be induced by proper selection of surfactants. CZTS nanocrystals having wurtzite (WZ) phase has also been synthesized by various methods such as noninjection (Regulacio et al. 2012), hot-injection (Singh et al. 2012) and hydrothermal method (Jiang et al. 2012). These processes require inert gas protection (nitrogen or argon), stepwise heating and relatively high temperature. Herein, we provide a facile noninjection synthetic route for preparing monodisperse anisotropic CZTS nanocones that adopt a WZ-type crystal structure. The noninjection or “heating up” approach for the formation of colloidal nanocrystals is better in terms of synthetic reproducibility, convenience in manipulating, and suitable for large-scale production compared to the hot-injection method. Optical properties of the grown nanocones were studied using UV–VIS spectrophotometer (Model: Shimadzu UV–VIS 1800). Structural properties were studied using powder XRD (Philips X’pert pro X-ray diffractometer) and Transmission electron microscopy (TEM, F30 S-twin 300 kV) was used to analyze the size and shape of the nanocrystals.
Experimental
For the synthesis of wurtzite CZTS nanocones, copper dithiocarbamates [Cu(dedtc)2] (36.0 mg, 0.1 mmol), zinc dithiocarbamates [Zn(dedtc)2] (18.1 mg, 0.05 mmol), tin dithiocarbamates [Sn(dedtc)4] (35.6 mg, 0.05 mmol), dodecanethiol (5 ml) and trioctylamine (5 ml) were taken in a 50-ml three-neck flask and the reaction mixture was degassed at 100 °C for 20–30 min. The clear yellow solution formed was heated to 250 °C under Ar atmosphere while stirring it vigorously. A change in color from yellow to topaz to dark brown was observed at around 220–240 °C. The stirring of resulting mixture was continued for 30 min while maintaining the 250 °C temperature. Then, it was placed in a H2O bath to allow the mixture to cool to 40 °C. Methanol was added to precipitate the nanocrystals. The nanocrystals are centrifuged. The solid obtained was washed thoroughly with methanol, and finally dissolved in chloroform.
Results and discussion
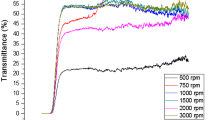
The transmittance spectra of the CZTS colloidal nanocones measured by the UV–VIS spectrometer in the wavelength range 300–1,400 nm are shown in Fig. 2a. Optical absorption spectra and Tauc’s plot of wurtzite-type Cu2ZnSnS4 colloidal nanocones are shown in Fig. 2b, c, respectively. The relation between absorption coefficient and optical absorbance can be given by.
where α is the absorption coefficient; ‘A’ is the optical absorbance, \(A = - \ln (\frac{I}{{I_{0} }})\); and ‘t’ is the thickness (the distance that light crosses in the medium). For direct band gap material (CZTS is a direct band gap material), the band gap is calculated using the equation.
where C is a constant, hν is the photon energy. The band gap (Eg) of CZTS nanocones can be estimated by plotting (αhν)2 versus hν, and extrapolating the linear portion of the X axis (hν). The estimated band gap value is 1.42 eV. The observed value is very close to the band gap reported in the literature for CZTS nanocrystals (Weber et al. 2009; Katagiri et al. 2009; Babu et al. 2010; Mitzi et al. 2011; Muhunthan et al. 2013). This value falls within the optimum band gap range for solar energy conversion in a single-junction device.
The powder X-ray diffraction (XRD) pattern of the as-obtained CZTS nanocones is shown in Fig. 3a. The diffraction pattern did not match with those reported in the literature for kesterite CZTS (JCPDS 26-0575). The diffraction peaks are attributed to (100), (002), (101), (102), (110), (103), (200), (112), (201) and (202) planes of wurtzite CZTS (Lu et al. 2011). The Raman spectra of the CZTS nanocones have a large peak at 331 cm−1, with additional small peaks at 286 and 363 cm−1. All these peaks are close to the value reported for bulk CZTS (Fig. 3b) (Fernandes et al. 2009). The broadening of the Raman peak for nanocrystals is due to the phonon confinement within the nanocrystals (Liu et al. 2013). No additional peaks of other phases such as ZnS, SnS and Cu2S, were observed which confirms the single-phase structure of CZTS nanocones.
Electron diffraction pattern, TEM and HRTEM images of CZTS nanocones are shown in Fig. 4a–d. The selected area electron diffraction pattern (SAED) shown in Fig. 4a confirms the crystalline nature of CZTS nanocones. Only WZ-type CZTS nanocones that are elongated in shape are observed in TEM images (Fig. 4b–c. The length of the cones is about 40 nm. The elongated nanocones with one end much wider (~13–15 nm) than the other (~5–8 nm) was observed. The nanocones are nearly monodispersed. The high-resolution TEM images (Fig. 4d) show a lattice spacing of 0.32 nm which corresponds to the (002) plane of wurtzite CZTS. The nanocone structure synthesized in the present study shall be useful for solar cell applications.
Conclusion
CZTS nanocones have been synthesized through a facile greener and inexpensive route, which involves the surfactant-assisted thermolysis of metal dithiocarbamates. Characterization using XRD, Raman shift and TEM confirm the phase purity of the CZTS nanocones. XRD and Raman shift measurements show that the CZTS nanocones have wurtzite structure. From optical absorption data, the band gap of the WZ-type CZTS nanocones is estimated to be 1.42 eV, which is optimal for solar cell applications. TEM studies show elongated nanocone structure with one end being wider (~13–15 nm) than the other (~5–8 nm). This nanocone structure should help in enhancing the efficiency of solar cell.
References
Babu GS, Kumar YBK, Bhaskar PU, Raja VS (2010) Effect of Cu/(Zn + Sn) ratio on the properties of co-evaporated Cu2ZnSnSe4 thin films. Sol Energ Mater Sol Cells 94:221–226
Chen S, Gong XG, Walsh A, Wei S-H (2009) Crystal and electronic band structure of CuZnSnX (X = S and Se) photovoltaic absorbers: first-principles insights. Appl Phys Lett 94:041903
Fan F-J, Wu L, Yu S-H (2014) Energetic I–III–VI2 and I2–II–IV–VI4 nanocrystals: synthesis, photovoltaic and thermoelectric applications. Energy Environ Sci 7:190–208
Fernandes PA, Salome PMP, da Cunha AF (2009) Growth and Raman scattering characterization of Cu2ZnSnS4 thin films. Thin Solid Films 517:2519–2523
Guo Q, Hillhouse HW, Agrawal R (2009) Synthesis of Cu2ZnSnS4 nanocrystal ink and its use for solar cells. J Am Chem Soc 131:11672–11673
Guo Q, Ford GM, Yang W-C, Walker BC, Stach EA, Hillhouse HW, Agrawal R (2010) Fabrication of 7.2 % efficient CZTSSe solar cells using CZTS nanocrystals. J Am Chem Soc 132:17384–17386
Hsu C-M, Connor ST, Tang MX, Cui Y (2008) Wafer-scale silicon nanopillars and nanocones by Langmuir–Blodgett assembly and etching. Appl Phys Lett 93:133109
Jiang H, Dai P, Feng Z, Fan W, Zhan J (2012) Phase selective synthesis of metastable orthorhombic Cu2ZnSnS4. J Mater Chem 22:7502–7506
Katagiri H, Jimbo K, Maw WS, Oishi K, Yamazaki M, Araki H, Takeuchi A (2009) Development of CZTS-based thin film solar cells. Thin Solid Films 517:2455–2560
Li M, Zhou W-H, Guo J, Zhou Y-L, Hou Z-L, Jiao J, Zhou Z-J, Du Z-L, Wu S-X (2012) Synthesis of pure metastable wurtzite CZTS nanocrystals by facile one-pot method. J Phys Chem C 116:26507–26516
Liu WC, Guo BL, Wu XS, Zhang FM, Mak CL, Wong KH (2013) Facile hydrothermal synthesis of hydrotropic Cu2ZnSnS4 nanocrystal quantum dots: band-gap engineering and phonon confinement effect. J Mater Chem A 1:3182–3186
Lu X, Zhuang Z, Peng Q, Li Y (2011) Wurtzite Cu2ZnSnS4 nanocrystals: a novel quaternary semiconductor. Chem Commun 47:3141–3143
Mainz R, Singh A, Levcenko S, Klaus M, Genzel C, Ryan KM, Unold T (2014) Phase-transition-driven growth of compound semiconductor crystals from ordered metastable nanorods. Nature Commun 5:3133
Mehta BR, Kruis FE (2005) A graded diameter and oriented nanorod-thin film structure for solar cell applications: a device proposal. Sol Energ Mater Sol Cells 85:107–113
Mitzi DB, Gunawan O, Todorov TK, Wang K, Guha S (2011) The path towards a high-performance solution-processed kesterite solar cell. Sol Energ Mater Sol Cells 95:1421–1436
Muhunthan N, Singh OP, Singh S, Singh VN (2013) Growth of CZTS thin films by co-sputtering of metal targets and sulfurization in H2S. Int J Photoenergy 2013:752012
Regulacio MD, Ye C, Lim SH, Bosman M, Ye E, Chen S, Xu Q-H, Han M-Y (2012) Colloidal nanocrystals of wurtzite-type Cu2ZnSnS4: facile noninjection synthesis and formation mechanism. Chem Eur J 18:3127–3131
Riha SC, Parkinson BA, Prieto AL (2009) Compositionally tunable Cu2ZnSn(S(1-x)Se(x))4 nanocrystals: probing the effect of Se-inclusion in mixed chalcogenide thin films. J Am Chem Soc 131:12054–12055
Shavel A, Arbiol J, Cabot A (2010) Synthesis of quaternary chalcogenide nanocrystals: stannite Cu(2)Zn(x)Sn(y)Se(1+x+2y). J Am Chem Soc 132:4514–4515
Singh A, Geaney H, Laffir F, Ryan KM (2012) Colloidal synthesis of wurtzite Cu2ZnSnS4 nanorods and their perpendicular assembly. J Am Chem Soc 134:2910–2913
Tanaka K, Moritake N, Uchiki H (2007) Preparation of Cu2ZnSnS4 thin films by sulfurizing sol–gel deposited precursors. Sol Energy Mater Sol Cells 91:1199–1201
Wang B, Leu PW (2012) Enhanced absorption in silicon nanocone arrays for photovoltaics. Nanotechnology 23:194003
Weber A, Krauth H, Perlt S, Schubert B, Kotschau I, Schorr S, Schock HW (2009) Multi-stage evaporation of Cu2ZnSnS4 thin films. Thin Solid Films 517:2524–2526
Yu Z, Fan S, Brongersma ML, McGehee MD, Cui Y (2012) Hybrid silicon nanocone–polymer solar cells. Nano Lett 12:2971–2976
Zhao Y, Qiao Q, Zhou W-H, Cheng X-Y, Kou D-X, Zhou Z-J, Wu S-X (2014) Wurtzite Cu2ZnSnS4 nanospindles with enhanced optical and electrical properties. Chem Phys Lett 592:144–148
Acknowledgments
V.N. Singh acknowledges Department of Science and Technology (DST) India for support through the fast track project for young scientist by sanction order no. SR/FTP/PS-124/2011 dated February, 2012. Authors are thankful to MNRE-India (sanction no. 31/29/2010-11/PVSE) for the financial support. The authors are grateful to CSIR-India for TAP-SUN program. OPS and NM are thankful to UGC for SRFships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Arora, L., Gupta, P., Chhikara, N. et al. Green synthesis of wurtzite copper zinc tin sulfide nanocones for improved solar photovoltaic utilization. Appl Nanosci 5, 163–167 (2015). https://doi.org/10.1007/s13204-014-0302-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0302-9