Abstract
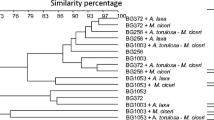
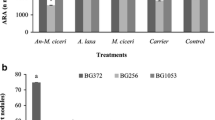
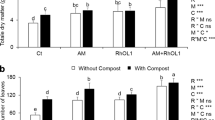
Chickpea (Cicer arietinum L.), a valuable legume crop, is an essential component of diet in many Asian, African, European and American countries. A major role in increasing its adaptive potential, quality and quantity of the yield is played by symbiotic and associative bacteria. The main aim of this research was to study the effect of combined pre-seed treatment with microbial inoculants and Mo nanoparticles on the composition of root exudates and the taxonomic diversity of microorganisms in the chickpea plants rhizosphere. We studied seed germination, the activity of enzymes involved in antioxidant protection system and the formation of plant-microbial system under the influence of nodule and rhizosphere bacteria and molybdenum (Mo) nanoparticles introduced into seeds before sowing. Rhizosphere microbiome was studied with the help of pyrosequencing method. Maximum numbers of introduced bacteria were observed in variants with Mo nanoparticles. The symbiotic effectiveness of Mesorhizobium ciceri strain ST282 was further improved by co-inoculation with “helper bacteria” Bacillus subtilis and Mo nanoparticles. Effective symbiosis was shown to be an important factor determining the formation of the rhizosphere microbiome by the representatives of the order Rhizobiales and influencing the numbers and the composition of the rhizosphere microbial community. A self-sufficient legume-rhizobium symbiosis improved the physiological status of the plant, increasing structural diversity of the microbial community of the rhizosphere through changes in the activity of root exudates, and paved the way for the development of the most effective associative bacteria.





Similar content being viewed by others
References
Andronov EE, Petrova CN, Chizevskaya EP, Korostik EV, Ahtemova GA, Pinaev AG (2009) Effect of introducing genetically modified strain of Sinorhizobium meliloti ACH-5 on the structure of soil microbial communities. Rus J Microbiol 78:525–534
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107
Barrett CF, Parker MA (2005) Prevalence of Burkholderia sp. nodule symbionts on four mimosoid legumes from Barro Colorado Island, Panama. Syst Appl Microbiol 28:57–65
Bates ST, Berg-Lyons JG, Caporaso WA (2010) Examining the global distribution of dominant archaeal populations in soil. ISME J 5:908–917
Bulgarelli D, Rott M, Schlaeppi K, Ver Loren van Themaat E, Ahmadinejad N, Assenza F (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95
Chebotar VK, Zavalin AA, Kiprushkina EI (2007) The effectiveness of a biological product ekstrasol. VNIIA, Moscow
Chen WM, Laevens S, Lee TM, Coenye T, De Vos P, Mergeay M, Vandamme P (2001) Ralstonia taiwanensissp. nov., isolated from root nodules of Mimosa species and sputum of a cystic fibrosis patient. Int J Syst Evol Microbiol 51:1729–1735
Chirak EL, Pershina EV, Dolnik AS, Kutovaya OV, Vasilenko ES, Kogut BM, Merzlyakova YV, Andronov EE (2013) Taxonomic structure of microbial association in different soils investigated by high-throughput sequencing of 16s-rrna gene library. Rus J Agr Biol 3:100–109
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:1. doi:10.1093/nar/gkn879
Dymov SI, Meek DJ, Steven B, Driscoll BT (2004) Insertion of transposon Tn5tac1 in the Sinorhizobium meliloti malate dehydrogenase (mhd) gene results in conditional polar effects on downstream TCA cycle genes. Mol Plant Microbe Interact 17:1318–1327
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. PNAS USA 103:626–631
Fierro-Coronado RA, Quiroz-Figueroa FR, García-Pérez LM, Ramírez-Chávez E, Molina-Torres J, Maldonado-Mendozaa IE (2014) IAA-producing rhizobacteria from chickpea (Cicer arietinum L.) induce changes in root architecture and increase root biomass. Can J Microbiol 60:639–648
Giri AP, Harsulkar AM, Patankar AG, Gupta VS, Sainani MN, Deshpande VV, Ranjekar PK (1998) Association of induction of protease and chitinase in chickpea roots with resistance to Fusarium oxysporum f.sp. ciceri. Plant Pathol 47:693–699
Gitte RR, Rai PV, Patil RB (1978) Chemotaxis of Rhizobium sp. towards root exudates of Cicer arietinum L. Plant Soil 50:553–566
Gonchar EN, Shcherbakov AV, Lopatko KG, Gonchar LN, Chebotar VK, Kalenska SM (2013) The efficiency improving of the microbiological preparations based on rhizospheric and endophytic bacteria through the creation of new compositions with nanosolutions of biogenic metals. Achiev Sci Techn AIC 12:30–35
Hynes RK, Leung GC, Hirkala DL, Nelson LM (2008) Isolation, selection, and characterization of beneficial rhizobacteria from pea, lentil and chickpea grown in Western Canada. Can J Microbiol 54:248–258
Jarvis BDW, Van Berkum P, Chen WX, Nour SM, Fernandez MP, Cleyet-Marel JC, Gillis M (1997) Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, and Rhizobium tianshanense to Mesorhizobium gen. nov. Int J Syst Bacteriol 47:895–898
Jones DG (1998) Role of legumes in sustainable cropping systems. In: Handarson G, Broughton G (eds) Molecular microbial ecology of the soil. Kluwer Academic Publishers, Dordrecht, pp 11–19
Kantar F, Hafeez FY, Shivakumar BG, Sundaram SP, Tejera NA, Aslam A, Bano A, Raja P (2007) Chickpea: Rhizobium management and nitrogen fixation. In: Yadav SS (ed) Chickpea breeding and management. Cromwell Press, Trowbridge, pp 179–192
Karr DB, Waters JK, Emerich DW, Emerich DW (1983) Analysis of poly-3-hydroxybutyrate in Rhizobium japonicum bacteroides by ion exclusion HPLC and UV detection. Appl Environ Microbiol 46:1339–1343
Karr DB, Waters JK, Suzuki F, Emerich DB (1984) Enzimes of poly-β-hydroxybutirate and citric acid cycles of Rhizobium japonicum bacteroides. Plant Physiol 75:2439–2469
Kathiravan R, Jegan S, Ganga V, Prabavathy VR, Tushar L, Sasikala C, Ramana CV (2013) Ciceribacter lividus gen. nov., sp. nov., isolated from rhizosphere soil of chick pea (Cicer arietinum L.). Int J Syst Evol Microbiol 63:4484–4488
Kravchenko LV, Azarova TS, Leonova-Erko EI, Tihonovich IA (2003) Root exudates of tomato and their impact on growth and antifungal activity of strains of Pseudomonas. Rus J Microbiol 72:48–53
Kuzmicheva YV, Shaposhnikov AI, Azarova TS, Petrova SN, Naumkina TS, Borisov AY, Belimov AA, Kravchenko LV, Parahin NV, Tihonovich IA (2014) The root exometabolites high effective pea varietie Triumph and its parent forms. Rus J Plant Physiol 61:121–128
Lee A, Hirsch AM (2006) Signals and responses: choreographing the complex interaction between legumes and α- and β-Rhizobia. Plant Signal Behav 4:161–168
Lopatko K, Aftandilyants EG, Tonkha AL, Kalenska SM (2009) Method of production of superdispersed powder. Ukraine patent № 38458
Lugtenberg B, Dekkers L, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Plant Pathol 39:461–490
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90
Malferrati G, Monferinin P, De Blasio P (2002) High-quality genomic DNA from human whole blood and mononuclear cells. Bio Tech 33:1228–1230
Mardis ER (2008) Next-generation DNA sequencing methods. Annu Rev Genomics Hum 9:387–402
McDermott TR, Kahn ML (1992) Cloning and mutagenesis of the Rhizobium meliloti isocitrate dehydrogenase gene. J Bacteriol 174:4790–4797
Mortimer MW, McDermott TR, York GM, Walker GC, Kahn ML (1999) Citrate synthase mutants of Sinorhizobium meliloti are ineffective and have altered cell surface polysaccharides. J Bacteriol 181:7608–7613
Nandasena KG, O’Hara GW, Tiwari RP, Willlems A, Howieson JG (2007) Mesorhizobium ciceri biovar biserrulae, a novel biovar nodulating the pasture legume Biserrula pelecinus L. Int J Syst Evol Microbiol 57:41–45
Nandwal AS, Kukreja S, Kumar N, Sharma PK, Jain M, Mann A, Singh S (2007) Plant water status, ethylene evolution, N2-fixing efficiency, antioxidant activity and lipid peroxidation in Cicer arietinum L. J Plant Physiol 164:1161–1169
Nascimento FX, Brígido C, Glick BR, Oliveira S, Alho L (2012) Mesorhizobium ciceri LMS-1 expressing an exogenous 1-aminocyclopropane-1-carboxylate (ACC) deaminase increases its nodulation abilities and chickpea plant resistance to soil constraints. Lett Appl Microbiol 55:15–21
Nemergut DR, Costello EK, Hamady M (2010) Global patterns in the biogeography of bacterial taxa. Environ Microbiol J 10:53–63
Odunfa SA, Werner D (1981) Root exudates in relation to growth and nitrogenase activity of Rhizobium japonicum. Z Allg Mikrobiol 21:601–606
Pershina EV, Andronov EE, Pinaev AG, Provorov NA (2013) Recent advances and Perspectives inmetagenomic studies of soil microbial communities. In: Malik A (ed) Management of microbial resources in the environment. Springer Science, Berlin, pp 141–164
Philips DA, Fox TC, King MD, Bhuwaneswari TV, Teuber LR (2004) Microbial products trigger amino acids exudation from plant roots. Plant Physiol 136:2887–2894
Rivas R, Gutierrez C, Abril A, Mateos PF, Martınez-Molina E, Ventosa A, Velazquez E (2005) Paenibacillus rhizosphaerae sp. nov., isolated from the rhizosphere of Cicer arietinum. Int J Syst Evol Microbiol 55:1305–1309
Romdhane BS, Tajini F, Trabelsi M, Aouani ME, Mhamdi R (2007) Competition for nodule formation between introduced strains of Mesorhizobium ciceri and the native populations of rhizobia nodulating chickpea (Cicer arietinum) in Tunisia. World J Microbiol Biotechnol 23:1195–1201
Ronaghi M (2004) Pyrosequencing: a tool for DNA sequencing analysis. Methods Mol Biol 255:211–219
Simons M, van der Bij AJ, Brand I, de Weger LA, Wij Velman CA, Lugtenberg BJ (1996) Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol Plant Microbe Interact 9:600–667
Singh R, Singh S, Parihar P, Mishra RK, Tripathi DK, Singh VP, Chauhan DK, Prasad SM (2016) Reactive oxygen species (ROS): beneficial companions of plants’ developmental processes. Front Plant Sci 7:1–19
Stevenson PC, Veitch NC (1996) Isoflavenes from the roots of Cicer judaicum. Phytochemistry 43:695–700
Stevenson PC, Veitch NC (1998) A 2-arylbenzofuran from roots of Cicer bijugum associated with fusarium wilt resistance. Phytochemistry 48:947–951
Stevenson PC, Padham DE, Phware M (1995) Root exudates associated with the resistance of four chickpea cultivars (Cicer arietinum) to two races of Fusarium oxysporum f. sp. ciceri. P Pathol 44:686–694
Stevenson PC, Turner HC, Haware MP (1997) Phytoalexin accumulation in the roots of chickpea (Cicer arietinumL.) seedlings associated with resistance to fusarium wilt (Fusarium oxysporumf.sp.ciceri). Physiol Mol Plant Pathol 50:167–178
Sugiyama A, Yazaki K (2012) Root exudates of legume plants and their involvement in interactions with soil microbes. In: Vivanco JM, Baluška F (eds) Secretions and exudates in biological systems, signaling and communication in plants. Springer, Berlin, pp 1227–1248
Tank N, Rajendran N, Patel B, Saraf M (2012) Evaluation and biochemical characterization of a distinctive pyoverdin from a pseudomonas isolated fromchickpea rhizosphere. Braz J Microbiol 43:639–648
Tarchevsky IA, Maksyutova NN, Yakovlev VG, Grechkin AN (1999) Succinic acid - salicylic acid mimetic. Russ J Plant Physiol 46:23–25
Tikhonovich IA, Provorov NA (2009) Symbiosis of plants and micro-organisms: molecular genetics agricultural systems of the future. SPBGU, St.Petersburg
Vancura V (1980) Fluorescent pseudomonads in the rhizosphere of plants and their relation to root exudates. Folia Microbiol 25:168–173
Vladimirov YA, Archakov AI (1972) Lipid peroxidation of biological membranes. Nauka, Moskow
Yarosh NP, Arasimovich VV, Ermakov AI, Peruvian JV (1987) Determination of the activity of enzymes and their inhibitors. In: Ermakov IA (ed) Methods of biochemical research of plants. Agropromizdat, Leningrad, pp 41–43
Acknowledgements
We thank Dr. S. V. Bulynsev (N.I. Vavilov Research Institute of Plant Industry, Russia) for providing us with chickpea seeds and Dr. K.G. Lopatko (National University of Life and Environmental Sciences of Ukraine) for providing us with CSM. Special thanks are due to Dr. A. Shaposhnikov (ARRIAM) for assistance in the study of root exudates. This study was partially financially supported by the Government of the Russian Federation, grant 074-U01, and performed using the equipment of the Centre for Collective Use “Genomic Technologies and Cell Biology” (ARRIAM). The work of A.V. Shcherbakov and V.K. Chebotar was supported by the Russian Scientific Fund (project no. 14-16-00146).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shcherbakova, E.N., Shcherbakov, A.V., Andronov, E.E. et al. Combined pre-seed treatment with microbial inoculants and Mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis 73, 57–69 (2017). https://doi.org/10.1007/s13199-016-0472-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-016-0472-1