Abstract
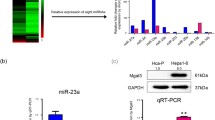
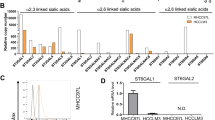
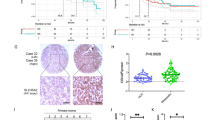
Glycosylation of cell surface proteins regulates critical cellular functions, including invasion and metastasis in cancer cells. Emerging evidence has shown that microRNAs (miRNAs) are involved in regulating both the glycosylation modifications on cell surface and the progression of cancer. In this study, we investigated the role of miR-9 in α-2,6-linked sialylation and the metastasis of mouse hepatocellular carcinoma (HCC). According to array-based miRNA expression profiling data of HCC cell lines Hepa1–6, Hca-P, and Hca-F with different lymphatic metastatic capacities, reverse correlation was found between miR-9 expression levels and the metastatic potential in these HCC cells. Additionally, β-galactoside α-2,6-sialyltransferase 1 (St6gal1) expression level is associated negatively with miR-9 and positively with metastatic potential. Bioinformatics analysis indicated that miR-9 could target St6gal1, which was verified by luciferase reporter assays. miR-9 overexpression reduced expression of St6gal1, which subsequently suppressed HCC cells metastatic potential. Moreover, upregulation of miR-9 could inhibit integrin-β1/FAK-mediated cell motility and migration signaling in mouse HCC cells. Together, our results suggest that miR-9 could act as a tumor suppressor and regulate mouse HCC cells migration and invasion by inhibiting the α-2,6-linked sialylation. This finding may provide insight into the relationship between abnormal miRNA expression and aberrant cell surface glycosylation during tumor lymphatic metastasis.





Similar content being viewed by others
References
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Britain CM, Holdbrooks AT, Anderson JC, Willey CD, Bellis SL (2018) Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J Ovarian Res 11:12
Chen X, Wang L, Zhao Y, Yuan S, Qiang W, Zhu X, Niang B, Wang S, Zhang J (2016) ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget 7:51955–51964
Deng B, Wang B, Fang J, Zhu X, Cao Z, Lin Q, Zhou L, Sun X (2016) MiRNA-203 suppresses cell proliferation, migration and invasion in colorectal cancer via targeting of EIF5A2. Sci Rep 6:28301. https://doi.org/10.1038/srep28301
Dennis JW, Granovsky M, Warren CE (1999) Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta Gen Subj 1473:21–34
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6:259–269
Fu X, Meng Z, Liang W et al (2014) miR-26a enhances miRNA biogenesis by targeting Lin28B and Zcchc11 to suppress tumor growth and metastasis. Oncogene 33:4296–4306
Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, Shi GM, Gao Q, Wang XY, Song K, Fan J, Ding ZB (2017) MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett 412:108–117. https://doi.org/10.1016/j.canlet.2017.10.012
Gao Y, Liu T, Huang Y (2015) MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and Notch pathway proteins. FEBS Lett 589:207–214
González-Vallinas M, Molina S, Vicente G, Zarza V, Martín-Hernández R, García-Risco MR, Fornari T, Reglero G, De Molina AR (2014) Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer. PLoS One 9:e98556
Guo Y, Li S, Qu J, Wang S, Dang Y, Fan J, Yu S, Zhang J (2011) MiR-34a inhibits lymphatic metastasis potential of mouse hepatoma cells. Mol Cell Biochem 354:275–282
Guo Y, Li S, Qu J, Ye L, Wang S, Fan J, Wang Q, Zhang J (2014) Let-7c inhibits metastatic ability of mouse hepatocarcinoma cells via targeting mannoside acetylglucosaminyltransferase 4 isoenzyme A. Int J Biochem Cell Biol 53:1–8
Higashi T, Hayashi H, Ishimoto T, Takeyama H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H (2015) miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in hepatocellular carcinoma cells. Br J Cancer 113:252–258
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ (2011) Biological functions of microRNAs: a review. J Physiol Biochem 67:129–139. https://doi.org/10.1007/s13105-010-0050-6
Hung WC, Chiang CH (2017) Up-regulation of sialyltransferases increases lymphatic metastasis in pancreatic cancer via the integrin-mediated pathway. Pancreatology 17:S19
Jia L, Wang S, Zhou H, Cao J, Hu Y, Zhang J (2006) Caveolin-1 up-regulates CD147 glycosylation and the invasive capability of murine hepatocarcinoma cell lines. Int J Biochem Cell Biol 38:1584–1593
Jungjin Park ML (2013) Increasing the α-2,6-sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver 7:629–641
Krichevsky AM, Sonntag KC, Isacson O, Kosik KS (2006) Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 24:857–864
Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW (2004) Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117:69–81
Lehmann U, Hasemeier B, Christgen M, Müller M, Römermann D, Länger F, Kreipe H (2008) Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J Pathol 214:17–24
Li Y, Qiu C, Tu J, Geng B, Yang J, Jiang T, Cui Q (2013) HMDD v2. 0: a database for experimentally supported human microRNA and disease associations. Nucleic Acids Res 42(D1):D1070–D1074
Li T, Dong ZR, Guo ZY, Wang CH, Zhi XT, Zhou JW, Li DK, Chen ZT, Chen ZQ, Hu SY (2015) Mannose-mediated inhibitory effects of PA-MSHA on invasion and metastasis of hepatocellular carcinoma via EGFR/Akt/IκBβ/NF-κB pathway. Liver Int 35:1416–1429
Schultz MJ, Swindall AF, Wright JW, Sztul ES, Landen CN, Bellis SL (2013) ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J Ovarian Res 6:25
Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL (2005) Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res 65:4645
Seguin L, Desgrosellier JS, Weis SM, Cheresh DA (2015) Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 25:234
Shah MH, Telang SD, Shah PM, Patel PS (2008) Tissue and serum α2-3- and α2-6-linkage specific sialylation changes in oral carcinogenesis. Glycoconj J 25:279–290
Stokes JB, Adair SJ, Slackdavis JK, Walters DM, Tilghman RW, Hershey ED, Lowrey B, Thomas KS, Bouton AH, Hwang RF (2011) Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther 10:2135–2145
Tan HX, Qian W, Chen LZ, Huang XH, Chen JS, Fu XH, Cao LQ, Chen XL, Wen L, Zhang L (2010) MicroRNA-9 reduces cell invasion and E-cadherin secretion in SK-Hep-1 cell. Med Oncol 27:654
Zhao Y, Li Y, Ma H, Dong W, Zhou H, Song X, Zhang J, Jia L (2014) Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol Cell Proteomics 13:520–536
Funding
The authors would like to gratefully acknowledge support from the Natural Science Foundation of China (21502015, 31570802) and the Fundamental Research Funds for the Central Universities (DUT17JC21, DUT18ZD208, DUT18LAB09).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 957 kb)
Rights and permissions
About this article
Cite this article
Han, Y., Liu, Y., Fu, X. et al. miR-9 inhibits the metastatic ability of hepatocellular carcinoma via targeting beta galactoside alpha-2,6-sialyltransferase 1. J Physiol Biochem 74, 491–501 (2018). https://doi.org/10.1007/s13105-018-0642-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-018-0642-0