Abstract
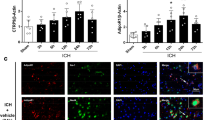
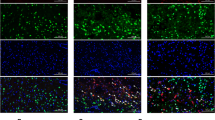
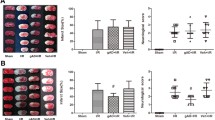
Intracerebral hemorrhage (ICH) is a life-threatening subtype of cerebral stroke with high morbidity and mortality; however, effective treatment for ICH is still lacking. Adiponectin (APN) is a a kind of fat-derived plasma protein with beneficial effects in cerebrovascular disorders. In this study, we aimed to investigate the protective effects of recombinant APN peptide (APNp) on brain injury after ICH in adult male C57BL/6J mice and further explored the underlying molecular mechanisms of these effects. APNp treatment exerted dose-dependent neuroprotective effects including improved neurological function, decreased brain edema, reduced neural apoptosis, and alleviated blood–brain barrier (BBB) disruption in ICH mice. We found the massive accumulation of APNp on reactive astrocytes around brain microvessels under hemorrhage conditions by immunofluorescence analysis. mRNA sequencing (mRNA-seq) and transcriptome analysis indicated that APNp significantly attenuated the inflammatory response and mitochondrial respiratory dysfunction in astrocytes. Further study revealed that this process was, at least in part, reliant on the inhibition of Drp1-mediated excessive mitochondrial fission. More specifically, APNp increased AMP-activated protein kinase (AMPK) activation-dependent Drp1 serine 637 (S637) phosphorylation, which inhibited the translocation of Drp1 to the mitochondrial membrane and reduced mitochondrial fragmentation and the production of mitochondrial superoxide, ultimately attenuating inflammatory brain injury induced by hemorrhage. In conclusion, we propose APNp as a potential therapeutic agent for ICH. We provide the first mechanistic evidence that APNp can modulate Drp1-mediated mitochondrial fission, which then contributes to alleviating astrocyte-derived inflammation.







Similar content being viewed by others
Change history
28 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12975-022-01085-6
References
Al-Shahi Salman R, Frantzias J, Lee RJ, Lyden PD, Battey TWK, Ayres AM, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17(10):885–94. https://doi.org/10.1016/s1474-4422(18)30253-9.
Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. Jama. 2015;314(9):904–12. https://doi.org/10.1001/jama.2015.10082.
Qureshi AI. Intracerebral hemorrhage specific intensity of care quality metrics. Neurocrit Care. 2011;14(2):291–317. https://doi.org/10.1007/s12028-010-9453-z.
Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, et al. Melatonin alleviates Intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl Stroke Res. 2018;9(1):74–91. https://doi.org/10.1007/s12975-017-0559-x.
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178:101610. https://doi.org/10.1016/j.pneurobio.2019.03.003.
Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13(7):420–33. https://doi.org/10.1038/nrneurol.2017.69.
Zhang P, Wang T, Zhang D, Zhang Z, Yuan S, Zhang J, et al. Exploration of MST1-mediated secondary brain injury induced by intracerebral hemorrhage in rats via hippo signaling pathway. Transl Stroke Res. 2019;10:729–43. https://doi.org/10.1007/s12975-019-00702-1.
Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585(23):3798–805. https://doi.org/10.1016/j.febslet.2011.08.033.
Li M, Li Z, Yao Y, Jin WN, Wood K, Liu Q, et al. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc Natl Acad Sci U S A. 2017;114(3):E396–e405. https://doi.org/10.1073/pnas.1612930114.
Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38(5):655–64. https://doi.org/10.1016/j.freeradbiomed.2004.11.021.
Min KJ, Yang MS, Kim SU, Jou I, Joe EH. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26(6):1880–7. https://doi.org/10.1523/jneurosci.3696-05.2006.
Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92(4):463–77. https://doi.org/10.1016/j.pneurobio.2010.08.001.
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–9. https://doi.org/10.1038/nature01705.
Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26(12):3226–9. https://doi.org/10.2337/diacare.26.12.3226.
Masamoto Y, Arai S, Sato T, Yoshimi A, Kubota N, Takamoto I, et al. Adiponectin enhances antibacterial activity of hematopoietic cells by suppressing bone marrow inflammation. Immunity. 2016;44(6):1422–33. https://doi.org/10.1016/j.immuni.2016.05.010.
Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115(11):1408–16. https://doi.org/10.1161/circulationaha.106.666941.
Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. Adiponectin: from obesity to cardiovascular disease. Obes Rev. 2009;10(3):269–79. https://doi.org/10.1111/j.1467-789X.2009.00571.x.
Ilhan N, Susam S, Canpolat O, Belhan O. The emerging role of leptin, adiponectin and visfatin in ischemic/hemorrhagic stroke. Br J Neurosurg. 2019:1–4. https://doi.org/10.1080/02688697.2019.1578862.
Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke. 2005;36(9):1915–9. https://doi.org/10.1161/01.STR.0000177874.29849.f0.
Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117(2):216–23. https://doi.org/10.1161/circulationaha.107.725044.
Qiao L, Kinney B, Yoo HS, Lee B, Schaack J, Shao J. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes. 2012;61(6):1463–70. https://doi.org/10.2337/db11-1475.
Wang S, Li D, Huang C, Wan Y, Wang J, Zan X, et al. Overexpression of adiponectin alleviates intracerebral hemorrhage-induced brain injury in rats via suppression of oxidative stress. Neurosci Lett. 2018;681:110–6. https://doi.org/10.1016/j.neulet.2018.05.050.
Miyatake Y, Shiuchi T, Ueta T, Taniguchi Y, Futami A, Sato F, et al. Intracerebroventricular injection of adiponectin regulates locomotor activity in rats. J Med Investig. 2015;62(3–4):199–203. https://doi.org/10.2152/jmi.62.199.
Rak A, Mellouk N, Froment P, Dupont J. Adiponectin and resistin: potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction. 2017;153(6):R215–r26. https://doi.org/10.1530/rep-17-0002.
Ujiie H, Oritani K, Kato H, Yokota T, Takahashi I, Maeda T, et al. Identification of amino-terminal region of adiponectin as a physiologically functional domain. J Cell Biochem. 2006;98(1):194–207. https://doi.org/10.1002/jcb.20779.
Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005–10. https://doi.org/10.1073/pnas.041591798.
Li X, Guo H, Zhao L, Wang B, Liu H, Yue L, et al. Adiponectin attenuates NADPH oxidase-mediated oxidative stress and neuronal damage induced by cerebral ischemia-reperfusion injury. Biochim Biophys Acta Mol basis Dis. 2017;1863(12):3265–76. https://doi.org/10.1016/j.bbadis.2017.08.010.
Lyzogubov VV, Tytarenko RG, Thotakura S, Viswanathan T, Bora NS, Bora PS. Inhibition of new vessel growth in mouse model of laser-induced choroidal neovascularization by adiponectin peptide II. Cell Biol Int. 2009;33(7):765–71. https://doi.org/10.1016/j.cellbi.2009.04.013.
Wu B, Ma Q, Khatibi N, Chen W, Sozen T, Cheng O, et al. Ac-YVAD-CMK decreases blood-brain barrier degradation by inhibiting caspase-1 activation of interleukin-1beta in intracerebral hemorrhage mouse model. Transl Stroke Res. 2010;1(1):57–64. https://doi.org/10.1007/s12975-009-0002-z.
Fang Y, Tian Y, Huang Q, Wan Y, Xu L, Wang W, et al. Deficiency of TREK-1 potassium channel exacerbates blood-brain barrier damage and neuroinflammation after intracerebral hemorrhage in mice. J Neuroinflammation. 2019;16(1):96. https://doi.org/10.1186/s12974-019-1485-5.
Liu H, Zhao L, Yue L, Wang B, Li X, Guo H, et al. Pterostilbene attenuates early brain injury following subarachnoid hemorrhage via inhibition of the NLRP3 inflammasome and Nox2-related oxidative stress. Mol Neurobiol. 2017;54(8):5928–40. https://doi.org/10.1007/s12035-016-0108-8.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–11. https://doi.org/10.1161/01.str.32.4.1005.
Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans blue injection routes: intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195(2):206–10. https://doi.org/10.1016/j.jneumeth.2010.12.013.
Zhao H, Zhang K, Tang R, Meng H, Zou Y, Wu P, et al. TRPV4 blockade preserves the blood-brain barrier by inhibiting stress fiber formation in a rat model of intracerebral hemorrhage. Front Mol Neurosci. 2018;11:97. https://doi.org/10.3389/fnmol.2018.00097.
Feng D, Guo B, Liu G, Wang B, Wang W, Gao G, et al. FGF2 alleviates PTSD symptoms in rats by restoring GLAST function in astrocytes via the JAK/STAT pathway. Eur Neuropsychopharmacol. 2015;25(8):1287–99. https://doi.org/10.1016/j.euroneuro.2015.04.020.
Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64(5):555–65. https://doi.org/10.1002/ana.21492.
Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15(2):186–200. https://doi.org/10.1016/j.cmet.2012.01.009.
Chen Z, Li Y, Wang Y, Qian J, Ma H, Wang X, et al. Cardiomyocyte-restricted low density lipoprotein receptor-related protein 6 (LRP6) deletion leads to lethal dilated cardiomyopathy partly through Drp1 signaling. Theranostics. 2018;8(3):627–43. https://doi.org/10.7150/thno.22177.
Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282(15):11521–9. https://doi.org/10.1074/jbc.M607279200.
Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22(2):256–65. https://doi.org/10.1091/mbc.E10-06-0551.
Wikstrom JD, Israeli T, Bachar-Wikstrom E, Swisa A, Ariav Y, Waiss M, et al. AMPK regulates ER morphology and function in stressed pancreatic beta-cells via phosphorylation of DRP1. Mol Endocrinol. 2013;27(10):1706–23. https://doi.org/10.1210/me.2013-1109.
Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105(41):15803–8. https://doi.org/10.1073/pnas.0808249105.
Desmoulins L, Chretien C, Paccoud R, Collins S, Cruciani-Guglielmacci C, Galinier A, et al. Mitochondrial dynamin-related protein 1 (DRP1) translocation in response to cerebral glucose is impaired in a rat model of early alteration in hypothalamic glucose sensing. Mol Metab. 2019;20:166–77. https://doi.org/10.1016/j.molmet.2018.11.007.
Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A, et al. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol. 2009;200(1):93–105. https://doi.org/10.1677/joe-08-0348.
Xu M, Wang L, Wang M, Wang H, Zhang H, Chen Y, et al. Mitochondrial ROS and NLRP3 inflammasome in acute ozone-induced murine model of airway inflammation and bronchial hyperresponsiveness. Free Radic Res. 2019;53(7):780–90. https://doi.org/10.1080/10715762.2019.1630735.
Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15(12):1126–33. https://doi.org/10.1038/ni.3015.
Kumari S, Anderson L, Farmer S, Mehta SL, Li PA. Hyperglycemia alters mitochondrial fission and fusion proteins in mice subjected to cerebral ischemia and reperfusion. Transl Stroke Res. 2012;3(2):296–304. https://doi.org/10.1007/s12975-012-0158-9.
Ye Xi, Dayun Feng, Kai Tao, Ronglin Wang, Yajun Shi, Huaizhou Qin, Michael P. Murphy, Qian Yang, Gang Zhao, (2018) MitoQ protects dopaminergicneurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1α. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1864 (9):2859-2870
Funding
This work was supported by grants from the State Key Program of the National Natural Science Foundation of China (81630027, to Dr. Qu), the National Natural Science Foundation of China (81571215, to Dr. Qu), and the Chang Jiang Scholar Program of China (to Dr. Qu).
Author information
Authors and Affiliations
Contributions
Y. Q. and D-Y. F. carried out this project and designed the study. X. W. performed the animal experiments, J-N. L. and H-X. L. performed the in vitro experiments, and W-X. C. and K. G. carried out statistical analysis and interpreted the data. L. Z. and H. B. helped in drafting the manuscript. H. G. and W. G. revised the manuscript. Thanks to Liang Jialong who help us to stimulate the structure diagram of APNp with Discovery Studio V16.1 software
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
All experimental procedures described here were approved by the Ethics Committee of the Fourth Military Medical University and performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Luo, J., Liu, H. et al. Recombinant Adiponectin Peptide Ameliorates Brain Injury Following Intracerebral Hemorrhage by Suppressing Astrocyte-Derived Inflammation via the Inhibition of Drp1-Mediated Mitochondrial Fission. Transl. Stroke Res. 11, 924–939 (2020). https://doi.org/10.1007/s12975-019-00768-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00768-x