Abstract
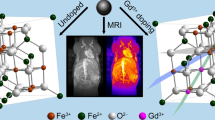
Several colloidal nanosystems have been developed aiming biomedical applications that include investigations related to magnetic resonance imaging (MRI) procedures. In this work, a core-shell structure consisting of iron oxide nanoparticles (IONPs) nucleus grafted with polyethylene glycol (PEG)4000 was prepared and evaluated by physicochemical and magnetic characterization. The polymer was covalently immobilized over nanoparticles through a simple and rapid in situ chemical method. The resulting hybrid material was capable of inducing a relevant T2 relaxation time in an average compared to marketed MRI formulations, revealing both physical characteristics and magnetic properties adequate for MRI applications. Furthermore, the in vitro biocompatibility profile was assessed by flow cytometry technique, using healthy human cell lineage (HEK-293). The results indicated good biocompatibility profile and non-anti-proliferative properties, and therefore, IONP-PEG can be considered as a new system candidate as a T2 contrast agent for MRI applications.









Similar content being viewed by others
References
Sudame, A., Kandasamy, G., & Maity, D. (2019). Single and dual surfactants coated hydrophilic superparamagnetic iron oxide nanoparticles for magnetic fluid hyperthermia applications. Journal of Nanoscience and Nanotechnology, 19, 3991–3999. https://doi.org/10.1166/jnn.2019.16326.
Xie, J., Chen, K., Huang, J., Lee, S., Wang, J., Gao, J., Li, X., & Chen, X. (2010). PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials, 31, 3016–3022. https://doi.org/10.1016/j.biomaterials.2010.01.010.
Kandasamy, G., & Maity, D. (2015). Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. International Journal of Pharmaceutics, 496, 191–218. https://doi.org/10.1016/J.IJPHARM.2015.10.058.
Wei, Y., Zhao, M., Yang, F., Mao, Y., Xie, H., & Zhou, Q. (2016). Iron overload by superparamagnetic iron oxide nanoparticles is a high risk factor in cirrhosis by a systems toxicology assessment. Scientific Reports, 6, 1–11. https://doi.org/10.1038/srep29110.
Lawaczeck, R., Menzel, M., & Pietsch, H. (2004). Superparamagnetic iron oxide particles: contrast media for magnetic resonance imaging. Applied Organometallic Chemistry, 18(10), 506–513. https://doi.org/10.1002/aoc.753.
De Montferrand, C., Hu, L., Milosevic, I., Russier, V., Bonnin, D., Motte, L., & Lalatonne, Y. (2013). Iron oxide nanoparticles with sizes, shapes and compositions resulting in different magnetization signatures as potential labels for multiparametric detection. Acta Biomaterialia, 9(4), 6150–6157. https://doi.org/10.1016/j.actbio.2012.11.025.
Che Rose, L., Bear, J. C., McNaughter, P. D., et al. (2016). A SPION-eicosane protective coating for water-soluble capsules: Evidence for on-demand drug release triggered by magnetic hyperthermia. Scientific Reports, 6, 20271. https://doi.org/10.1038/srep20271.
Janko, C., Zaloga, J., Pöttler, M., Dürr, S., Eberbeck, D., Tietze, R., & Alexiou, C. (2017). Strategies to optimize the biocompatibility of iron oxide nanoparticles—“SPIONs safe by design”. Journal of Magnetism and Magnetic Materials, 431, 281–284. https://doi.org/10.1016/J.JMMM.2016.09.034.
Jose, G., Lu, Y.-J., Chen, H.-A., Hsu, H.-L., Hung, J.-T., Anilkumar, T. S., & Chen, J.-P. (2019). Hyaluronic acid modified bubble-generating magnetic liposomes for targeted delivery of doxorubicin. Journal of Magnetism and Magnetic Materials, 474, 355–364. https://doi.org/10.1016/j.jmmm.2018.11.019.
Chervanyov, A. I., & Heinrich, G. (2007). Flory radius of polymers in a periodic field: an exact analytic theory. European Physical Journal E: Soft Matter and Biological Physics, 24, 271–276. https://doi.org/10.1140/epje/i2007-10237-9.
Jaiswal, M. K., Pradhan, L., Vasavada, S., De, M., Sarma, H. D., Prakash, A., & Dravid, V. P. (2015). Magneto-thermally responsive hydrogels for bladder cancer treatment: therapeutic efficacy and in vivo biodistribution. Colloids and Surfaces. B, Biointerfaces, 136, 625–633. https://doi.org/10.1016/j.colsurfb.2015.09.058.
Ishida, T., Harada, M., Wang, X. Y., Ichihara, M., Irimura, K., & Kiwada, H. (2005). Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. Journal of Controlled Release, 105, 305–317. https://doi.org/10.1016/j.jconrel.2005.04.003.
Owens, D. E., & Peppas, N. (2006). Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics, 307, 93–102. https://doi.org/10.1016/j.ijpharm.2005.10.010.
Suk, J. S., Xu, Q., Kim, N., Hanes, J., & Ensign, L. M. (2016). PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Advanced Drug Delivery Reviews, 99, 28–51. https://doi.org/10.1016/j.addr.2015.09.012.
Yang, Q., Jones, S. W., Parker, C. L., Zamboni, W. C., Bear, J. E., & Lai, S. K. (2014). Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Molecular Pharmaceutics, 11, 1250–1258. https://doi.org/10.1021/mp400703d.
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A., & Chan, W. C. W. (2012). Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. Journal of the American Chemical Society, 134, 2139–2147. https://doi.org/10.1021/ja2084338.
de Gennes, P. G. (1980). Conformations of polymers attached to an Interface. Macromolecules, 13, 1069–1075. https://doi.org/10.1021/ma60077a009.
Yang, Q., & Lai, S. K. (2015). Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology, 7, 655–677. https://doi.org/10.1002/wnan.1339.
Lopes, T. C. M., Silva, D. F., Costa, W. C., Frézard, F., Barichello, J. M., Silva-Barcellos, N. M., Lima, W. G., & Rezende, S. A. (2018). Accelerated blood clearance (ABC) phenomenon favors the accumulation of tartar emetic in pegylated liposomes in BALB/c mice liver. Pathology Research International, 2018, 1–7. https://doi.org/10.1155/2018/9076723.
Wang, L., Su, Y., Wang, X., Liang, K., Liu, M., Tang, W., Song, Y., Liu, X., & Deng, Y. (2017). Effects of complement inhibition on the ABC phenomenon in rats. Asian Journal of Pharmaceutical Sciences, 12, 250–258. https://doi.org/10.1016/J.AJPS.2016.06.004.
Kang, M. K., Mao, W., & Yoo, H. S. (2018). Surface-initiated atom transfer radical polymerization of cationic corona on iron oxide nanoparticles for magnetic sorting of macrophages. Biomaterials Science, 6, 2248–2260. https://doi.org/10.1039/c8bm00418h.
Wu, W., Wu, Z., Yu, T., Jiang, C., & Kim, W.-S. (2015). Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Science and Technology of Advanced Materials, 16, 023501. https://doi.org/10.1088/1468-6996/16/2/023501.
Wu, W., Jiang, C. Z., & Roy, V. A. L. (2016). Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale, 8, 19421–19474. https://doi.org/10.1039/c6nr07542h.
Gates-Rector, S., & Blanton, T. (2019). The powder diffraction file: a quality materials characterization database. Powder Diffraction, 34, 352–360. https://doi.org/10.1017/s0885715619000812.
Zhang, B., Tu, Z., Zhao, F., & Wang, J. (2013). Superparamagnetic iron oxide nanoparticles prepared by using an improved polyol method. Applied Surface Science, 266, 375–379. https://doi.org/10.1016/j.apsusc.2012.12.032.
Ali, S., Khan, S. A., Eastoe, J., Hussaini, S. R., Morsy, M. A., & Yamani, Z. H. (2018). Synthesis, characterization, and relaxometry studies of hydrophilic and hydrophobic superparamagnetic Fe3O4 nanoparticles for oil reservoir applications. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 543, 133–143. https://doi.org/10.1016/j.colsurfa.2018.02.002.
Park, J. Y., Daksha, P., Lee, G. H., Woo, S., & Chang, Y. (2008). Highly water-dispersible PEG surface modified ultra-small superparamagnetic iron oxide nanoparticles useful for target-specific biomedical applications. Nanotechnology, 19(36), 365603. https://doi.org/10.1088/0957-4484/19/36/365603.
Kim, W., Suh, C. Y., Cho, S. W., Roh, K. M., Kwon, H., Song, K., & Shon, I. J. (2012). A new method for the identification and quantification of magnetite–maghemite mixture using conventional X-ray diffraction technique. Talanta, 94, 348–352. https://doi.org/10.1016/j.talanta.2012.03.001.
Karimzadeh, I., Aghazadeh, M., Doroudi, T., Ganjali, M. R., & Kolivand, P. H. (2017). Superparamagnetic iron oxide (Fe3O4) nanoparticles coated with PEG/PEI for biomedical applications: a facile and scalable preparation route based on the cathodic electrochemical deposition method. Advances in Physical Chemistry, 2017, 1–7. https://doi.org/10.1155/2017/9437487.
Cooper, D. H., & Mooney, N. K. (2013). Convention on biological diversity. Encyclopedia of Biodiversity, 306–319. https://doi.org/10.1016/b978-0-12-384719-5.00418-4.
Sun, N., Chen, D.-X., Gu, H.-C., & Wang, X.-L. (2009). Experimental study on relaxation time of protons in water suspensions of iron-oxide nanoparticles: waiting time dependence. Journal of Magnetism and Magnetic Materials, 321, 2971–2975. https://doi.org/10.1016/j.jmmm.2009.04.073.
Cho, H. R., Choi, S. H., Lee, N., Hyeon, T., Kim, H., & Moon, W. K. (2012). Macrophages homing to metastatic lymph nodes can be monitored with ultrasensitive ferromagnetic Iron-oxide nanocubes and a 1.5T clinical MR scanner. PLoS One, 7, e29575. https://doi.org/10.1371/journal.pone.0029575.
Psimadas, D., Bouziotis, P., Georgoulias, P., Valotassiou, V., Tsotakos, T., & Loudos, G. (2013). Radiolabeling approaches of nanoparticles with99mTc. Contrast Media & Molecular Imaging, 8, 333–339. https://doi.org/10.1002/cmmi.1530.
Cho, K., Wang, X., Nie, S., Chen, Z., & Shin, D. M. (2008). Therapeutic nanoparticles for drug delivery in cancer. Clinical Cancer Research, 14, 1310–1316. https://doi.org/10.1158/1078-0432.ccr-07-1441.
Caldorera-Moore, M., Guimard, N., Shi, L., & Roy, K. (2010). Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opinion on Drug Delivery, 7, 479–495. https://doi.org/10.1517/17425240903579971.
Xu, C., Haque, F., Jasinski, D. L., Binzel, D. W., Shu, D., & Guo, P. (2018). Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer Letters, 414, 57–70. https://doi.org/10.1016/j.canlet.2017.09.043.
Feng, Q., Liu, Y., Huang, J., Chen, K., Huang, J., & Xiao, K. (2018). Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Scientific Reports, 8, 2082. https://doi.org/10.1038/s41598-018-19628-z.
Arami, H., Khandhar, A., Liggitt, D., & Krishnan, K. M. (2015). In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chemical Society Reviews, 44, 8576–8607. https://doi.org/10.1039/c5cs00541h.
Joseph, E., & Singhvi, G. (2019). Multifunctional nanocrystals for cancer therapy: a potential nanocarrier. In A. M. Grumezescu (Ed.), Nanomaterials for drug delivery and therapy (1st ed., pp. 91–116). Amsterdam: Elsevier. https://doi.org/10.1016/b978-0-12-816505-8.00007-2.
Mahdavi, M., Namvar, F., Ahmad, M., & Mohamad, R. (2013). Green biosynthesis and characterization of magnetic Iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules, 18, 5954–5964. https://doi.org/10.3390/molecules18055954.
Petcharoen, K., & Sirivat, A. (2012). Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Materials Science & Engineering, B: Solid-State Materials for Advanced Technology, 177, 421–427. https://doi.org/10.1016/j.mseb.2012.01.003.
Akal, Z. Ü., Alpsoy, L., & Baykal, A. (2016). Biomedical applications of SPION@APTES@PEG-folic acid@carboxylated quercetin nanodrug on various cancer cells. Applied Surface Science, 378, 572–581. https://doi.org/10.1016/j.apsusc.2016.03.217.
Dai, L., Liu, Y., Wang, Z., Guo, F., Shi, D., & Zhang, B. (2014). One-pot facile synthesis of PEGylated superparamagnetic iron oxide nanoparticles for MRI contrast enhancement. Materials Science and Engineering: C, 41, 161–167. https://doi.org/10.1016/j.msec.2014.04.041.
Almaki, J. H., Nasiri, R., Idris, A., Majid, F. A. A., Salouti, M., Wong, T. S., & Amini, N. (2016). Synthesis, characterization andin vitroevaluation of exquisite targeting SPIONs–PEG–HER in HER2+ human breast cancer cells. Nanotechnology, 27, 105601. https://doi.org/10.1088/0957-4484/27/10/105601.
Viali, W. R., da Silva Nunes, E., dos Santos, C. C., da Silva, S. W., Aragón, F. H., Coaquira, J. A. H., & Jafelicci, M. (2013). PEGylation of SPIONs by polycondensation reactions: a new strategy to improve colloidal stability in biological media. Journal of Nanoparticle Research, 15, 1824. https://doi.org/10.1007/s11051-013-1824-x.
Sabareeswaran, A., Ansar, E. B., Harikrishna, V. P. R. V., Mohanan, P. V., & Kumary, T. V. (2016). Effect of surface-modified superparamagnetic iron oxide nanoparticles (SPIONS) on mast cell infiltration: an acute in vivo study. Nanomed-Nanotechnol, 12, 1523–1533. https://doi.org/10.1016/j.nano.2016.02.018.
Thapa, B., Diaz-Diestra, D., Beltran-Huarac, J., Weiner, B. R., & Morell, G. (2017). Enhanced MRI T2 relaxivity in contrast-probed anchor-free PEGylated Iron oxide nanoparticles. Nanoscale Research Letters, 12, 312. https://doi.org/10.1186/s11671-017-2084-y.
Schweiger, C., Pietzonka, C., Heverhagen, J., & Kissel, T. (2011). Novel magnetic iron oxide nanoparticles coated with poly(ethylene imine)-g-poly(ethylene glycol) for potential biomedical application: synthesis, stability, cytotoxicity and MR imaging. International Journal of Pharmaceutics, 408, 130–137. https://doi.org/10.1016/j.ijpharm.2010.12.046.
Park, Y. C., Smith, J. B., Pham, T., Whitaker, R. D., Sucato, C. A., Hamilton, J. A., & Wong, J. Y. (2014). Effect of PEG molecular weight on stability, T2 contrast, cytotoxicity, and cellular uptake of superparamagnetic iron oxide nanoparticles (SPIONs). Colloids and Surfaces. B, Biointerfaces, 119, 106–114. https://doi.org/10.1016/j.colsurfb.2014.04.027.
Liu, D., Wu, W., Ling, J., Wen, S., Gu, N., & Zhang, X. (2011). Effective PEGylation of iron oxide nanoparticles for high performance in vivo cancer imaging. Advanced Functional Materials, 21, 1498–1504. https://doi.org/10.1002/adfm.201001658.
Al Faraj, A. (2013). Preferential magnetic nanoparticle uptake by bone marrow derived macrophages sub-populations: effect of surface coating on polarization, toxicity, and in vivo MRI detection. Journal of Nanoparticle Research, 15, 1797. https://doi.org/10.1007/s11051-013-1797.
Singh, A., Verma, N., & Kumar, K. (2019). Hybrid composites: a revolutionary trend in biomedical engineering. In V. Grumezescu & A. M. Grumezescu (Eds.), Materials for biomedical engineering: bioactive materials, properties, and applications (1st ed., pp. 33–46). Elsevier, Amsterdam. https://doi.org/10.1016/b978-0-12-818431-8.00002-7.
Sarasini, F. (2017). Low-velocity impact behaviour of hybrid composites. In V. K. Thakur & M. K. Thakur (Eds.), Pappu A hybrid polymer composite materials: Properties and characterisation (1st ed., pp. 151–168). Elsevier, Woodhead Publishing. https://doi.org/10.1016/b978-0-08-100787-7.00007-x.
Funding
The authors received financial support from the CNPq (405749/2016-3) and FAPEMIG (PPM-00125-18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barcelos, K.A., Tebaldi, M.L., do Egito, E.S.T. et al. PEG-Iron Oxide Core-Shell Nanoparticles: In situ Synthesis and In vitro Biocompatibility Evaluation for Potential T2-MRI Applications. BioNanoSci. 10, 1107–1120 (2020). https://doi.org/10.1007/s12668-020-00791-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-020-00791-5