Abstract
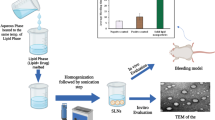
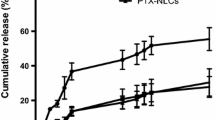
The aim of the present study is to develop the melphalan (MLN) loaded stealth solid lipid nanoparticles (SSLNs) for parenteral delivery by hot homogenization technique using central composite design. Amount of tristearin, soyalecithin, and DSPE-m-PEG-2000 is taken as independent factor whereas the particle size, % of encapsulation efficiency, and zeta potential are considered as dependent factors. Developed SSLNs were characterized through various physicochemical parameters, i.e., percentage of drug content, particle size, zeta potential, polydispersity, and percent encapsulation efficiency. The SSLNs were optimized using desirability and overlay plots, and optimized SSLNs were further characterized for various physicochemical parameters, i.e., in vitro drug release at pH 7.4, 6.5 and 5.5 by dialysis process, drug and excipient compatibility analysis (FTIR, DSC, TGA, SEM, and XRD), sterilization, in vitro cell viability and hematological studies, in vivo pharmacokinetics on rats, and stability, respectively. The % assay was found in the range 97.89 ± 0.9 to 101 ± 1.6%; particle size was found in the range of 106 ± 0.9 to 301 ± 1.2 nm whereas the zeta potential was found in the range of − 4 to -38 mV, respectively. The drug release of SSLN was found at around 100% at 28, 32, and 34 h for pH 7.4, 6.5 and 5.5 mediums, respectively. The lyophilized form of SSLNs showed better stability compared with SSLN suspension. The in vitro cell viability studies indicated that SSLNs showed superior results compared with their pure MLN solution. The in vivo pharmacokinetic studies revealed that SSLNs showed long circulation (t1/2), better residence time (MRT), and low elimination rate (Kel) compared with pure MLN solution. Melphalan loaded stealth solid lipid nanoparticles were successfully designed using central composite design by high-pressure homogenization with low particle size and high encapsulation efficiency (92%) and are able to release the drug in controlled manner.










Similar content being viewed by others
References
Wu, X. Z. (2006). A new classification system of anticancer drugs based on cell biological mechanisms. Med Hypotheses, 66(5), 883–887. https://doi.org/10.1016/j.mehy.2005.11.036.
Das, M., Mohanty, C., & Sahoo, S. K. (2009). Ligand-based targeted therapy for cancer tissue. Expert Opin Drug Deliv, 6(3), 285–304. https://doi.org/10.1517/17425240902780166.
Casonova, F., Quarti, J., da Costa, D. C., Ramos, C. A., da Silva, J. L., & Fialho, E. (2012). Resveratrol chemosensitizes breast cancer cells to melphalan by cell cycle arrest. J Cell Biochem, 113(8), 2586–2596. https://doi.org/10.1002/jcb.24134.
https://www.drugbank.ca/drugs/DB01042. Accessed on 05/07/2016
https://www.rxlist.com/alkeran-drug.htm. Accessed on 05/07/2016
Yingchoncharoen, P., Kalinowski, D. S., & Richardson, D. R. (2016). Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev, 68(3), 701–787. https://doi.org/10.1124/pr.115.012070.
Gregoriadis, G. (2018). Liposomology: delivering the message. J Liposome Res, 28(1), 1532–2394. https://doi.org/10.1080/08982104.2018.1429356.
Gabizon, A. A. (2001). Stealth liposomes and tumor targeting: one step further in the quest for magic Bullet. Clin. Cancer Res, 7(2), 223–225 https://www.ncbi.nlm.nih.gov/pubmed/11234871.
Lucks, S., & M & s:, R. R.. (1999a). Medication vehicles made of solid lipid particles (solid lipid nanospheres-SLN),EP0605497B2.(1999a). https://patents.google.com/patent/EP0605497B2/hr
Muhlen Zur, A., Schwarz, C., & Mehnert, W. (1998). Solid lipid nanoparticles (SLN) for controlled drug delivery-drug release and release mechanism. Eur J Pharm Biopharm, 45(2), 149–155. https://doi.org/10.1016/S0939-6411(97)00150-1.
Cerpnjak, K., Zvonar, A., Gasperlin, M., & Vrecer, F. (2013). Lipid-based systems as promising approach, for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharma, 63(4), 427–445. https://doi.org/10.2478/acph-2013-0040.
Fahr, A., & Liu, X. (2007). Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv, 4(4), 403–416. https://doi.org/10.1517/17425247.4.4.403.
Bunies, H. (2010). Lipid nanoparticles for the delivery of poorly water-soluble drugs. J Pharm Pharmacol, 62(11), 1637–1645. https://doi.org/10.1111/j.2042-7158.2010.01024.x.
Fufundaro, A., Cavalli, R., Bargoni, A., Vighetto, D., Zara, G. P., & Gasco, M. R. (2000). Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol Res, 42(4), 337–343. https://doi.org/10.1006/phrs.2000.0695.
Kuznetsova, N. R., Stepanova, E. V., Peretolchina, N. M., Khochenkov, D. A., Boldyrev, I. A., Bovin, N. V., & Vodovozova, E. L. (2014). Targeting liposomes loaded with melphalan prodrug to tumour vasculature via the sialyl Lewis X selectin ligand. J Drug Target, 22(3), 242–250. https://doi.org/10.3109/1061186X.2013.862805.
Chelvi, T. P., & Ralhan, R. (1997). Hyperthermia potentiates antitumor effect of thermosensitive-liposome-encapsulated melphalan and radiation in murine melanoma. Tumor Biol, 18(4), 250–260 https://www.ncbi.nlm.nih.gov/pubmed/9218010.
Stella, B., Peira, E., Dianzani, C., Gallarate, M., Battaglia, L., Gigliotti, C. L., Boggio, E., Dianzani, U., & Dosio, F. (2018). Development and characterization of solid lipid nanoparticles loaded with a highly active doxorubicin derivative. Nanomaterials, 8(2), 110. https://doi.org/10.3390/nano8020110.
Raju, K. K., Sudhakar, B., & Ramana Murthy, K. V. (2014). Factorial design studies and biopharmaceutical evaluation of simvastatin loaded solid lipid nanoparticles for improving the oral bioavailability. ISRN Nanotechnology Article ID, 951016, 1–8. https://doi.org/10.1155/2014/951016.
Bodratti, A., & Alexandridis, P. (2018). Formulation of poloxamers for drug delivery. J Funct Biomater, 9(1), 11. https://doi.org/10.3390/jfb9010011.
Singh, R., & Lillard Jr., J. W. (2009). Nanoparticle-based targeted drug delivery. Exp Mol Pathol, 86(3), 215–223. https://doi.org/10.1016/j.yexmp.2008.12.004.
Mehnert, W., & Mander, K. (2001). Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev, 64, 83–101. https://doi.org/10.1016/j.addr.2012.09.021.
Zhao, Y., Wang, L., Yan, M., et al. (2012). Repeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beagles. Int J Nano medicine, 7, 2891–2900. https://doi.org/10.2147/IJN.S30943.
Brightman, K., Finlay, G., Jarvis, I., Knowlton, T., & Manktelow, C. T. (1999). A stability-indicating method for the determination of melphalan and related impurity content by gradient HPLC. J Pharm Pharm Sci, 20(3), 439–447. https://doi.org/10.1016/S0731-7085(99)00011-4.
Pinguet, F., Joulia, J. M., Martel, P., Grosse, P. Y., Astre, C., & Bressolle, F. (1996). High-performance liquid chromatographic assay for melphalan in human plasma. Application to pharmacokineticstudies, J Chromatogr B, 686(1), 43–49. https://doi.org/10.1016/S0378-4347(96)00184-3.
Varshosaz, J., Ghaffari, S., Khoshayand, M. R., Atyabi, F., Azarmi, S., & Kobarfard, F. (2010). Development and optimization of solid lipid nanoparticles of amikacin by central composite design. J Liposome Res, 20(2), 97–104. https://doi.org/10.3109/08982100903103904.
Sudhakar, B., Krishna, M. C., & Murthy, K. V. R. (2016). Factorial design studies of antiretroviral drug-loaded stealth liposomal injectable PEGylation, lyophilization and pharmacokinetic studies. Appl Nanosci, 6(1), 43–60. https://doi.org/10.1007/s13204-015-0408-8.
Arjun, N., & Veerabrahma, K. (2013). Preparation characterization and evaluation of quetiapine fumarate solid lipid nanoparticles to improve the oral bioavailability. J Pharm Sci Article ID, 265741, 1–7. https://doi.org/10.1155/2013/265741.
Wesley, N. O., Bernhards, O., Florence, O., Hulda Swai, L. K., Paula, M., Geoffrey, M. M., & Jeremiah, W. G. (2014). Preparation characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int J Nanomed, 9(1), 3865–3874. https://doi.org/10.2147/IJN.S62630.
Rosa, G. D., Stefano, M. D., Ungaro, F., & Rotonda, M. L. (2008). Cold field emission gun-scanning electron microscopy: new tool for morphological and ultrastructural analysis of liposomes. Int J Pharm, 362(1-2), 189–192. https://doi.org/10.1016/j.ijpharm.2008.06.003.
Costa, P., & Jose, M. S. L. (2001). Modeling and comparison of dissolution profiles. Eur J Pharm Sci, 13(2), 123–133. https://doi.org/10.1016/S0928-0987(01)00095-1.
Peppas, N. A. (1985). Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv, 60(4), 110–111 https://www.ncbi.nlm.nih.gov/pubmed/4011621.
Howard, M. D., Lu, X., Jay, M., & Dziubla, T. D. (2012). Optimization of the lyophilization process for long-term stability of solid-lipid nanoparticles. Drug Dev Ind Pharm, 38(10), 1270–1279. https://doi.org/10.3109/03639045.2011.645835.
Cherry, C. L. A., Millward, H., Cooper, R., & Landon, J. (2014). A novel approach to sterile pharmaceutical freeze-drying. Pharm Dev Technol, 19(1), 73–81. https://doi.org/10.3109/10837450.2012.752388.
Sanyog, J. S., Jagadish, M. S., Amit, K. J., & Rahul, R. M. (2013). Surface-stabilized lopinavir nanoparticles enhance oral bioavailability without coadministration of ritonavir. Nano medicine, 8(10), 1639–1655. https://doi.org/10.2217/nnm.12.181.
http://www.ich.org/products/guidelines/quality/quality-single/article/stability-testing-of-new-drug-substances-and-products.html. Accessed on 06/07/02017.
Mahesh, G. A., Narsireddy, A., Madhusudana Rao, N., & Manorama, V. S. (2018). A simple approach to design chitosan functionalized Fe3O4 nanoparticles for pH responsive delivery of doxorubicin for cancer therapy. J Magn Mag Mater, 448, 199–207. https://doi.org/10.1016/j.jmmm.2017.09.018.
Suelen, P. F., Debora, B. S., Pedro, V. A. B., Paulo, R. S., Luana, C. F., Heveline, D. M. F., Catia, S. N., Johny, P. M., Elton, G. B., Celso, V. N., Edvani, C. M., & Alessandro, F. M. (2016). Preparation and cytotoxicity of N-modified chitosan nanoparticles applied in curcumin delivery. Int J Biol Macromol, 87, 237–245. https://doi.org/10.1016/j.ijbiomac.2016.02.063.
Barbara, S., Elena, P., Chiara, D., Marina, G., Luigi, B., Casimiro, L. G., Elena, B., Umberto, D., & Franco, D. (2018). Development and characterization of solid lipid nanoparticles loaded with a highly active doxorubicin derivative. Nanomaterials, 8, 110. https://doi.org/10.3390/nano8020110.
Petterino, C., & Argentino, S. A. (2006). Clinical chemistry and haematolofy historical data in Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol, 57(3), 213–219. https://doi.org/10.1016/j.etp.2005.10.002.
Said, N. M., & Abiola, O. (2014). Haematological profile shows that inbred Sprague Dawley rats have exceptional promise for use in biomedical and pharmacological studies. Asian J Pharm, 4(37), 33–37. https://doi.org/10.15272/ajbps.v4i37.597.
Limit size lipid nanoparticles and related methods. US 2014/0328759 A1. https://patents.google.com/patent/US20140328759A1/en.
Garbuzenko, O., Zalipsky, S., Qazen, M., & Barenholz, Y. (2005). Electrostatics of PEGylated micelles and liposomes containing charged and neutral lipopolymers. Langmuir, 21(6), 2560–2568. https://doi.org/10.1021/la0479105.
Shlear, H. H., & Nabeel, S. Othman, Kafia M. Surchi. (2013) Solid lipid nanoparticles for topical delivery of meloxicam: development and in vitro characterization. European Scientific journal ESJ 3: 779-798. https://doi.org/10.19044/esj.2013.v9n21p%25p.
Syed, A. A. R., & Saleh, A. M. (2018). Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J, 26(1), 64–70. https://doi.org/10.1016/j.jsps.2017.10.012.
Zur, M. A., Schwarz, C., & Mehnert, W. (1998). Solid lipid nanoparticles (SLN) for controlled drug delivery—drug release and release mechanism. Eur J Pharm Biopharm, 45(2), 149–155. https://doi.org/10.1016/S0939-6411(97)00150-1.
https://www.pda.org/.../defining-a-strategy-for-the-validation-and-qualification. Accessed on 23/07/2019.
Patravale, V. B., & Ambarkhane, A. V. (2003). Study of solid lipid nanoparticles with respect to particle size distribution and drug loading. Pharmazie, 58(6), 392–395 https://www.ncbi.nlm.nih.gov/pubmed/12857001.
George, G. B., & Parker, K. (2003). Understanding the complete blood count with differential. J PeriAnesth Nurs, 18(2), 96–117. https://doi.org/10.1053/jpan.2003.50013.
Chow, P. K., Robert, T. H. N., & Bryan, E. O. (2008). Using animal models in biomedical research, the rationale for the use of animal models in Bio medical research. World scientific, 308. https://doi.org/10.1142/6454.
Peng., Z., Lawrence, H., Anirban, M., Stephan, T. S., & Scott, E. M. (2013). Polymeric curcumin nanoparticle pharmacokinetics and metabolism in bile duct cannulated rats. Mol Pharm, 10(5), 1977–1987. https://doi.org/10.1021/mp4000019.
Ihedioha, J. I., Okafor, C., & Ihedioha, T. E. (2004). The haematological profile of the Sprague-Dawley outbred albino rat in Nsukka, Nigeria. Animal Research International, 1(2), 125–132. https://doi.org/10.4314/ari.v1i2.40755.
Availability of data and materials
Available upon the request
Author information
Authors and Affiliations
Contributions
KVNSR is the main author of the study; BS is involved in designing the graphs, and VSAPR performed the experiments, interpreted the data, and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The protocol for the in vivo experiments has been approved by the Institute of Animal Ethics Committee (IAEC) of CPCSEA, India (Protocol No. Ref. No/IAEC/XII/01/SIPS/2017-2018).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rudhrabatla, V.S.A.P., Sudhakar, B. & Reddy, K.V.N.S. In Vitro and In Vivo Assessment of Designed Melphalan Loaded Stealth Solid Lipid Nanoparticles for Parenteral Delivery. BioNanoSci. 10, 168–190 (2020). https://doi.org/10.1007/s12668-019-00680-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-019-00680-6