Abstract
This study analysed the effect of a mixture of pethoxamid (P) and terbuthylazine (T) contained in the herbicide Successor T 550 SE on organotrophic bacteria, total oligotrophic bacteria, Azotobacter and actinomycetes, oligotrophic sporulating bacteria, fungi and on the activities of dehydrogenases, catalase, urease, alkaline phosphatase, acid phosphatase, arylsulphatase and β-glucosidase. The phytotoxic effect of this pesticide on maize was also determined. The study was undertaken because of a lack of data concerning the effect of a P+T mixture on soil metabolism. The previously undertaken studies concerned only the separate effect of each of these substances. The P+T mixture disturbed soil homeostasis and altered soil stability, resulting in a succession of K-strategy organotrophic bacteria. It had a negative effect on bacteria of the genus Azotobacter, oligotrophic sporulating bacteria, actinomycetes and fungi, and a positive effect on oligotrophic bacteria. P+T in doses greater than 0.73 mg kg−1 of soil resulted in a strong inhibition of dehydrogenases, catalase, urease, acid phosphatase, alkaline phosphatase, arylsulphatase and β-glucosidase, and significantly inhibited the growth and development of maize.
Similar content being viewed by others
Introduction
In the last several decades, an increase has been observed in the use of chemical plant protection products in agriculture. Because of such wide use of pesticides, their remains can be found in various elements of the environment, namely in water, soil and the atmosphere (Riah et al. 2014). The time and degree of decomposition of pesticides in the environment depend on many physical and chemical soil factors (Arias-Estévez et al. 2008). Intensive use of pesticides may have serious ecological consequences, which may consequently have an effect on a cultivated plant and on soil microbiome (Kalia and Gosal 2011). However, the impact of plant protection products on microorganisms may have diverse effects. Some pesticides contribute to an increase in the microorganism count, while others may inhibit their development or have no effect (Jastrzębska and Kucharski 2007; Lo 2010). The effect of plant protection products on the soil microbiome largely depends on the dose and type of product used and on the type of the microorganisms (Lone et al. 2014). Not only pesticides themselves pose a threat to the environment, but also their metabolites.
Soil enzymes are a very good indicator of soil fertility and quality. They are very sensitive to various stress factors, including contaminants (Jyot et al. 2015). Enzymes such as: dehydrogenases, acid and alkaline phosphatase, β-glucosidase, urease and arylsulphatase participate in the turnover of the basic elements (phosphorus, carbon, nitrogen and sulphur) in the environment. For this reason, they play such an important role in the proper functioning of ecosystems (Riah et al. 2014). According to Floch et al. (2011), the activities of enzymes are the most credible and reliable indicator for assessing the effect of pesticides on a soil environment.
Pethoxamid [2-chloro-N-(2-ethoxyethyl)-N-(2-phenylprop-1-enyl-2-methyl) acetamide] is a compound belonging to the chloroacetamides. This substance is often used to combat weeds in maize and soybean (Kato et al. 2001). Its half-life is from 6.1 to 14.2 days (Jursík et al. 2013). Terbuthylazine [2-tert-butylamine-4-chloro-6-ethylamine-1,3,5-triazine] is a substance introduced in place of atrazine (Caracciolo et al. 2005). It belongs to the group of s-triazine herbicides. In the EU countries, it has been successfully used for over 10 years (Jurina et al. 2014). According to the literature data (Dousset et al. 1997; James et al. 1998; Sahid and Teoh 1994), the half-life of terbuthylazine is from 5 to 116 days. The half-life is closely connected with the type of soil and the temperature.
In the literature (Caracciolo et al. 2005; Dousset et al. 1997; James et al. 1998; Jursík et al. 2013; Sahid and Teoh 1994; Skrzypczak et al. 2007), reports can be found concerning the herbicidal effectiveness or degradation in soil of both studied substances, but only of them used separately. However, there is no information concerning the synergic or additive effect of either of these substances on soil microorganisms, enzymatic activity or the growth and development of plants. It was precisely this lack of information that prompted the need for a study to unequivocally define the interaction of pethoxamid and terbuthylazine on soil microorganisms, soil enzymatic activity and the yielding of maize.
Materials and methods
Characteristics of soil
The research material was soil collected in the Research and Education Centre in Tomaszkowo (the NE part of Poland, 53.71610 N, 20.41670 E), from a depth of 0 to 20 cm, from the arable–humus horizon. Tomaszkowo is located in the area of the Olsztyn Lakeland which constitutes the western part of the Masurian Lakeland. The object is located within the Pomeranian phase of the Main Stadial of the Vistulian Glaciation. In this landscape, ground moraine prevails, where the dominant type of soils is brown soils with crude soils and leached brown soils. In this part of the region, areas used for agriculture prevail. The soil used for the study, according to the WRB classification (World Reference Base for Soil Resources 2014), was classified as Eutric Cambisols. Ratio C:N-8:1. The physicochemical properties of the soil are shown in Table 1.
Description of experiment
The next stage of the study was to conduct a pot culture experiment in the vegetation hall of the University of Warmia and Mazury in Olsztyn. In each of 3.5 dm3 polyethylene pots, 3 kg of air dry mass of soil were placed. The experiment was conducted in five repetitions. Before being placed in the pot, the soil was thoroughly mixed with macro- and microelements. For all objects, the same fertilisation level was used, in mg kg−1 of soil: N—200 (100 mg—before sowing and 100 mg at the BBCH 19 phase of maize), P—44, K—100, Mg—20, Cu—5, Zn—5, Mo—5, Mo—2.5 and B—0.33. Nitrogen was used in the form of CO(NH2)2, phosphorus—KH2PO4, potassium—KH2PO4 + KCl, magnesium—MgSO4·7H2O, copper—CuSO4 · 5H2O, zinc—ZnSO4, manganese—MnCl2 · 4H2O, molybdenum—NaMoO4 · 5H2O and boron—H3BO3. To the prepared soil, a mixture of pethoxamid and terbuthylazine was added, in amounts of: 0; 0.73; 14.63; 29.26; 58.52; 117.04; 234.08; 468.16 mg kg−1 of DM of soil. The control object was soil without the herbicide added. The soil was then brought to moisture equal to 50 % capillary water capacity, and maize of the variety LG 32.58 was sown. After the emergence phase, thinning of plants was carried out to leave only five plants in each pot. The plants were vegetated for 60 days. The maize was harvested at the BBCH 53 phase (the top of the ear can be seen). Microbiological and biochemical analyses were performed twice during the plant vegetation (on days 30 and 60). The date of performance of microbiological and biochemical analyses was determined based on the PEC results Table 2. Therefore, microbiological and biochemical tests on the soil were performed twice during the vegetation of crop plants, in two different maize development phases, i.e. with nine leaves unfolded (30th day of the experiment—BBCH 19 phase) and the tip of inflorescence emerged (60th day of the experiment—BBCH 53 phase).
Characteristics of the herbicide
In the pot culture experiment, the herbicide Successor T 550 SE was used which contained two active substances: pethoxamid (300 g dm−3) and terbuthylazine (250 g dm−3). The predicted environmental concentrations (PEC) in the soil of these substances are presented in Table 2. Predicted environmental concentrations (PEC) were determined with the use of the below formula: PEC = PECinitial × (1−e−kt)/kt, PECinitial—predicted concentration of preparation in soil after single application (mg kg−1 of soil); k−ln2/DT50 (DT50—time for disappearance of half the chemical (days); t—time between applications (days) proposed by the European Comission—EC Document UE.7617/VI/96 (FOCUS 1997).
Terbuthylazine (T) is a compound from the group of triazines. It is taken up by roots of weeds. Pethoxamid (P) is a compound from the group of chloroacetamides. It is taken up by seedlings. Successor T 550 SE is produced by the company Stähler International GmbH & Co. KG (Germany). The recommended dose of this herbicide is 4 dm3 ha−1, or 1.33 mm3 kg−1. The dose of the herbicide per 1 kg was calculated based on the soil bulk density, which was 1.5 g cm−3. Successor T 550 SE is a concentrate in the form of suspo-emulsion to be diluted with water. It is intended for combating weeds in maize crops.
Determination of the soil microorganism counts
On the days 30 and 60 of the experiment, the counts of organotrophic bacteria, total oligotrophic bacteria and oligotrophic sporulating bacteria, Azotobacter, actinomycetes and fungi were determined. In five repetitions, the respective dilutions of soil were sown onto Petri dishes. On the media characterised by Baćmaga et al. (2015).
Diversity of soil microorganisms
On days 30 and 60, the structure and biodiversity of organotrophic bacteria, actinomycetes and fungi were determined. The biodiversity of microorganisms was determined based on a 10-day observation and on daily counting of the grown microorganism colonies for each of the experimental days. Then, based on the formula proposed by Sarathachandra et al. (1997), the colony development index (CD) for the microorganisms was determined CD = [N1/1 + N2/2 + N3/3…N10/10] × 100, where N1, N2, N3,…N10 were proportional counts of colonies that emerged on day 1, 2, 3,…,10. According to the formula proposed by De Leij et al. (1993) the ecophysiological diversity index (EP) for the microorganisms was determined EP = −∑(pi × log pi) where pi—counts of colonies that emerged on a given day divided by total counts of colonies.
Determination of the soil enzyme activities
On the days 30 and 60 of the experiment, the activities of the following soil enzymes were determined: dehydrogenases (EC 1.1), catalase (EC1.11.1.6), urease (EC 3.5.1.5), arylsulphatase (EC 3.1.6.1), β-glucosidase (EC 3.2.1.21), acid phosphatase (EC 3.1.3.2) and alkaline phosphatase (EC 3.1.3.1). The exact assay procedure of enzyme activity is described in the publication Kucharski et al. (2016).
Statistical analysis of the results
All of the obtained results were statistically analysed using the Statistica 12.5 (StatSoft 2016) software package. The analysis of variance ANOVA was used using Duncan’s multiple range test, at a significance level of p = 0.05. Based on the obtained results, the percentage of the observed variation η 2 was determined using the two-way ANOVA method. The responses of microorganisms to the tested herbicide were compared using the cluster method—the Ward dendrogram. The activities of the soil enzymes were shown using principal component analysis (PCA) performed using multi-dimensional exploratory techniques.
Results and discussion
The counts of soil microorganisms
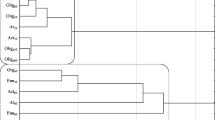
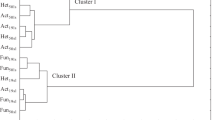
The use of herbicides may result in changes, both quantitative and qualitative, of microorganisms living in the soil environment (Vlad et al. 2012). The performed studies demonstrated that the count of soil microorganisms was determined by the dose of a mixture of pethoxamid (P) and terbuthylazine (T) within the range from 15.98 % (organotrophic bacteria) to 64.71 % (Azotobacter), and the duration of herbicide persistence in soil from 6.77 to 58.65 % (Table 3). The response of the microorganisms to P+T are displayed on a cluster analysis diagram, with the Ward method (Fig. 1). This method allows one to generalise and properly interpret the obtained results. It is aimed at the determination of a similar response of microorganisms to herbicide contamination. Three separate clusters were created consisting of several sub-clusters with homogeneous variances. The first cluster was formed by oligotrophic bacteria (the days 30 and 60), organotrophic bacteria (the day 60) and fungi (the day 30). The second cluster was formed with oligotrophic sporulating bacteria (days 30 and 60), organotrophic bacteria, bacteria of the genus Azotobacter, actinomycetes (the day 30), the third cluster: bacteria of the genus Azotobacter, actinomycetes and fungi (the day 60). Contamination of the soil with the tested herbicide had a significant effect on the count of microorganisms on both tested days. On both the 30th and 60th day of the experiment, the counts of oligotrophic spore-forming bacteria, Azotobacter, actinomycetes and fungi were negatively correlated with the P+T dose, while the count of oligotrophic bacteria was positively correlated. Apart from the duration of the experiment, it was found that soil contamination with P+T contributed to a decrease in the count of oligotrophic sporulating bacteria, Azotobacter, actinomycetes and fungi, and to an increase in total oligotrophic bacteria (Fig. 2). The response of oligotrophic bacteria different from other groups of microorganisms may result from different abilities of microorganisms to degrade plant protection products and from oligotrophic bacteria being microorganisms with low nutritional requirements. In addition, they have a membrane which constitutes a barrier against harmful environmental factors (HuiXia et al. 2007). An increase in the count of oligotrophic bacteria in soil supplemented with pethoxamid and terbuthylazine (the active substances of the herbicide Successor T 550 SE) was also observed in the previous studies by our team, conducted under strictly controlled conditions (Tomkiel et al. 2014). The response of microorganisms in the vegetation experiment which tested the effects of a mixture of pethoxamid and terbuthylazine was different from that in the laboratory model experiment, which is due to the fact that these are experiments of a different type. The experiment described in the presented manuscript is a vegetation experiment in which, as compared to the studies conducted previously under laboratory conditions, another element was introduced which affected the soil microbiota. This element was the cultivated crop plant. If the plant did not affect the soil microbiota, the commonly known rhizosphere effect (R:S ratio) would not be experienced. Thus, both our own studies and the literature (Baćmaga et al. 2015; Kucharski et al. 2016; Sebiomo et al. 2011) demonstrate that herbicides are not always toxic to soil microbiome. Some species or strains may display increased tolerance to herbicides. The differentiated response of microorganisms to the excessive content of herbicides in soil may result from both their species characteristics and morphological characteristics. The effect of herbicides on microbiological properties is dictated by the type of active substance, its dose and physicochemical properties of soil (Kucharski et al. 2016). For example: carfentrazone-ethyl (the active substance of the preparation Aurora 40 WG) had an effect on an increase in the counts of total oligotrophic bacteria and organotrophic bacteria, while it decreased the counts of Azotobacter, fungi, oligotrophic sporulating bacteria and actinomycetes (Tomkiel et al. 2015). Metazachlor had a negative effect on the development of oligotrophic bacteria and their spore forms, Azotobacter, organotrophic bacteria and actinomycetes (Baćmaga et al. 2014a). Atrazine, primextra, paraquat and glyphosate also had a negative effect on the development of bacteria, actinomycetes and fungi, according to Sebiomo et al. (2011). In contrast, Baćmaga et al. (2015) following the use of a mixture of active substances named diflufenican, mesosulphuron-methyl and iodosulphuron-methyl-sodium observed an increase in the counts of total oligotrophic bacteria and their spore forms, organotrophic bacteria and actinomycetes, and a decrease in the counts of fungi and Azotobacter. Martinez et al. (2008) studying sulfentrazone found that this product stimulates the growth of actinomycetes, but it does not have an effect on the count of fungi. Araújo et al. (2003) noted a significant increase in the count of actinomycetes following the application of glyphosate. Crouzet et al. (2010) studying mesotrione at increased doses (tenfold, 100-fold higher than the recommended) found an increase in the count of fungi. Milošević et al. (2004) and Elbashier et al. (2016) also noted the sensitivity of bacteria of the genus Azotobacter to pesticides. Thus, it can be clearly stated that the responses of microorganisms to contamination of a soil environment with herbicides are very diverse. It should also be noted that root exudates such as: carbohydrates, organic acids, amino acids, enzymes and flavonoids, accumulating in the maize rhizosphere had a significant effect on the responses of particular groups of microorganisms to a mixture of pethoxamid and terbuthylazine. Root exudates may increase the ability of microorganisms to degrade plant protection products and increase their bioavailability.
Diversity of soil microorganisms
The common use of pesticides in plant protection has an effect not only on a change in the count of autochthonous microorganisms in soil, but it can cause physiological and biochemical changes in soil microorganisms and change their diversity (Kucharski et al. 2016). In our own studies, in order to determine the diversity of soil microorganisms, the colony development index (CD) and the ecophysiological diversity index (EP) of microorganisms were computed. The CD and EP indices provide information concerning changes in the proportions between the slow-growing (K-strategists) and the fast-growing (r-strategists) microorganisms. The CD index ranges from 0 to 100, and the EP index from 0 to 1. If the CD values are close to 100, it indicates the dominance of the fast-growing microorganisms and if the EP values are close to 1, then the growth of microorganisms in a given environment is more even.
In the conducted studies, pethoxamid and terbuthylazine to a slight degree changed the ecophysiological diversity index of microorganisms (Table 4). Of all the studied microorganisms, the lowest EP values were found in fungi. Low values of this index may indicate that sensitive microorganisms were replaced with microorganisms more resistant to a stress factor (in this case, the tested herbicide). For fungi, both on the day 30 and 60, the (EP) index value was highest for soil to which a mixture P+T was applied, in an amount of 0.73 mg kg−1. The EP index was 0.623 and 0.588, respectively. For organotrophic bacteria, the EP values were highest for objects contaminated with a dose of P+T in the amount of 29.26 mg kg−1. This was observed for the whole duration of the study (60 days). The ecophysiological diversity index of bacteria on day 30 of herbicide persistence in soil was 0.898, and on day 60 it was 0.933. The EP index of actinomycetes on day 30 had the highest values following the application of the preparation in a dose of 14.63 mg P+T kg−1 (EP = 0.889) and on day 60 following the use of the product in the amount of 58.52 mg P+T kg−1 (EP = 0.917).
The tested herbicide contributed to changes in the value of the colony development index (CD) of all the three studied groups of microorganisms (Table 5). Regardless of the dose of herbicide or the time of analysis, the highest values of the colony development index CD were noted in fungi (CD = 40.116), organotrophic bacteria (CD = 34.395) and the lowest values were in actinomycetes (CD = 24.566). The colony development index CD of fungi varied from 32.155 to 48.759. In fungi, the lowest values of this index were noted following the application of the two highest doses of P+T (234.08 and 468.16 mg kg−1). The CD values for actinomycetes were within the range from 20.201 (a dose of 58.52 mg kg−1 on day 30) to 32.853 (the control object on day 60). In organotrophic bacteria on day 30, the highest values of this index were noted following the application of a dose of 234.08 mg kg−1 (CD = 37.063) and on day 60 in the control object (CD = 43.219). The greatest changes in the CD index value occurred in organotrophic bacteria, after relatively slight fluctuations between objects on day 30. On day 60, a drastic change occurred in the growth rate of the colonies. The rate decreased with an increasing dose of P+T. The CD index for organotrophic bacteria on the 30th day of the experiment increased following the application of doses from 58.52 to 234.08 mg kg−1 DM soil as compared to the control sample, while on the 60th day, the addition of the tested herbicide resulted in a decrease in this index. The CD index for actinomycetes on both days decreased following the application of the herbicide in an amount from 14.63 to 468.16 mg kg−1 DM soil. In turn, the CD index for fungi on the 30th day decreased following the application of the tested preparation as compared to the control object, while on the 60th day, the trend was reversed. To conclude, it can be stated that the values of the CD and EP indices prove that the tested herbicide Successor T 550 SE had an effect on the structure of microorganisms. The changes in diversity of microorganisms, observed in our studies, in soil supplemented with herbicide, are concordant with the literature data (Kucharski et al. 2016; Lone et al. 2014; Ratcliff et al. 2006). Organotrophic bacteria, as opposed to fungi, were characterised by the greatest diversity in soil with an addition of the herbicide Boreal 58 WG (flufenacet + isoxaflutole) (Kucharski et al. 2016) and a mixture of diflufenican + mesosulphuron-methyl + iodosulphuron-methyl-sodium (Baćmaga et al. 2015). In contrast, in studies using herbicides Alister Grande 190 OD (diflufenican + mesosulphuron-methyl + iodosulphuron-methyl-sodium), Fuego 500 SC (matazachlor) and Lumax 537,5 SC (terbuthilazine + mesatrione + s-metolachlor) it was observed that the values of the ecophysiological diversity index EP of fungi decreased, but the colony development index CD increased (Baćmaga et al. 2014b). Following the use of preparations soproturon, metribuzin, clodinafop propargyl, atlantis and sulfosulfuron, Lone et al. (2014) observed their differentiated effect on the biodiversity of microorganisms.
The activities of soil enzymes
Due to their protein nature, enzymes secreted to the environment by microorganisms, plants and soil fauna respond quickly to various environmental factors, both natural and anthropogenic. For this reason, enzymes are among the most important indicators determining soil quality (Kucharski et al. 2016). The enzymes actively participating in the turnover of elements, the transformations of organic matter and transformation of xenobiotics entering soil include, among others, dehydrogenases, catalase, urease, acid phosphatase, alkaline phosphatase, arylsulphatase and β-glucosidase. Because of new plant protection products coming onto the market all the time, their effect on the activities of soil enzymes has not yet been sufficiently explained. In our own studies, it was demonstrated that the activities of enzymes (Table 6) were shaped by the dose of the P+T mixture within a range from 14.95 % (arylsulphatase) to 90.56 % (urease), and by the duration of herbicide persistence in soil from 0.11 % (dehydrogenases) to 74.51 % (arylsulphatase). The data presented in Table 7 clearly show that a dose of 0.73 mg P+T kg−1 already destabilised the activities of most enzymes. When the substances were used in higher doses (from 14.63 to 468.16 mg P+T kg−1 of soil), strong inhibition of all enzymes was found. On average, regardless of the time of study, in most contaminated soil (a dose of 468.16 mg P+T mg kg−1 of soil) the mixture caused inhibition of enzymatic activity within a range from 21 % (catalase) to 90 % (dehydrogenases). In terms of sensitivity to a P+T mixture, enzymes can be ordered as follows (from the most to the least sensitive): dehydrogenases > acid phosphatase > urease > alkaline phosphatase > β-glucosidase > arylsulphatase > catalase. Principal component analysis (PCA) indicates not only a negative correlation between the activities of particular enzymes and the degree of soil contamination with pethoxamid and terbuthylazine, but also a significant positive correlation of enzymes between each other (Fig. 3). The first two components together constituted 83.03 % of the total variation. The vectors representing the primary variables of the activities of catalase, alkaline phosphatase, dehydrogenases and urease were negatively correlated with both the first and the second principal component, and the vectors representing the activities of arylsulphatase, β-glucosidase and acid phosphatase were negatively correlated with the first principal component, but positively with the second principal component. It is not an isolated fact that contamination of soil on which plants are cultivated with a P+T mixture, results in disturbances in soil metabolism. The unfavourable effect of these substances, though tested under strictly controlled laboratory conditions, was observed by Tomkiel et al. (2015). Kucharski et al. (2016) and Wyszkowska (2002) also noted the differentiated effect of herbicides on enzyme activities. Kucharski et al. (2016) demonstrated that contamination with the herbicide Boreal 58 WG (40 mg kg−1 of soil) contributed to a decrease in the activities of dehydrogenases, catalase, urease, arylsulphatase and β-glucosidase, but the preparation did not have a negative effect on the acid phosphatase activity.
Enzyme activity in soil with the Pethoxamid (P) and Terbuthylazine (T)—PCA method. Deh dehydrogenases, Ure urease, Cat catalase, Pal alkaline phosphatase, Pac acid phosphatase, Aryl arylosulfatase, Glu β-glucosidase, dose of P+T (mg kg−1 DM soil): 1–0 mg; 2–0.732 mg; 3–14.630 mg; 4–29.260 mg; 5–58.520 mg; 6–117.04 mg; 7–234.08 mg; 8–468.16; soil incubation time, days: 30 and 60
The preparation Reglone 200 SL (diquat) resulted in an increase in the activities of dehydrogenases, acid phosphatase and alkaline phosphatase, and Elastiq 550 EC (synthetic latex + alkoxylated alcohol)—in a decrease in their activities (Jezierska-Tys and Rutkowska 2013). The sensitivity of dehydrogenases to atrazine, primextra, paraquat and glyphosate was also demonstrated by Sebiomo et al. (2011). Similar results were obtained by Lone et al. (2014), testing six different herbicides (soproturon, metribuzin, clodinafop propargyl, atlantis and sulfosulfuron). They proved that dehydrogenases were the most sensitive to the used preparations. Acid phosphatase responded more negatively to these products than alkaline phosphatase. A similar result was noted in our studies with P+T. A negative effect on phosphatases was also demonstrated by Yao et al. (2006) when testing acetamiprid and by Wyszkowska and Kucharski (2004) testing Triflurotox 250 EC (trifluranine).
Baćmaga et al. (2014a) demonstrated a negative effect of metazachlor on the activities of dehydrogenases, catalase, urease, acid and alkaline phosphatase, arylsulphatse and β-glucosidase, and Muñoz-Leoz et al. (2012) found a negative effect of tebuconazole on the β-glucosidase activity. In contrast, Saha et al. (2012), testing chloroacetanilide herbicides (alachlor, butachlor and pretilachlor), observed a stimulating effect of the preparations on the β-glucosidase activity. In our own studies, the β-glucosidase activity was not disturbed before a dose of P+T higher than 0.73 mg kg−1 of soil was used.
The inhibitory effect of the P+T mixture used in excessive doses on the activities of the tested soil enzymes may result from not only a direct inhibitory effect of these substances, but also from the effect of intermediary products formed during the decomposition of pethoxamid and terbuthylazine. The negative effect of this herbicide may also depend on the duration of its persistence in a soil environment (Kucharski et al. 2016). The authors demonstrated that, over time, in soil treated with the herbicide Boreal 58 WG, the activities of urease and catalase decreased, and the activities of acid phosphatase and β-glucosidase increased. Also, the P+T mixture (the object of this study) resulted in changes in the activities of soil enzymes during the experiment. With the increased duration of persistence in soil of pethoxamid and terbuthylazine, the activities of dehydrogenases, catalase, urease and alkaline phosphatase decreased and the activities of acid phosphatase, arylsulphatase and β-glucosidase increased. Based on the presented predicted environmental concentrations (PEC) (Table 2) of the active substances, it can be stated that terbuthylazine had a greater effect on the activities of soil enzymes than pethoxamid. It was demonstrated that on day 60 of the experiment, following the application of the highest dose of the tested preparation, the predicted concentration of pethoxamid in soil was 13.652 mg kg−1 and terbuthylazine was 33.238 mg kg−1. In conclusion, it can be stated that both microorganisms and enzymes are very good potential indicators of soil quality. This results mainly from them being more sensitive to stress and responding more quickly to contamination of the environment with biocides than other parameters.
Growth and development of maize
Soil contamination with a mixture of pethoxamid and terbuthylazine changed not only the soil microbiome, but also had a significant effect on the growth and development of maize (Fig. 4). The key factor determining the growth and development of maize was the dose of the applied herbicide. Only the dose of herbicide recommended by the producer did not significantly disturb plant growth. Skrzypczak et al. (2007), trying to determine the herbicidal effectiveness of a mixture of two herbicides, Callisto 100 SC and Successor T 550 SE, applied in different doses (150 + 1650; 100 + 1650; 100 + 1375 g ha−1) also did not find a phytotoxic effect on maize.
In the presented studies, a feedback was observed between the degree of soil contamination with the tested product and the yield of plants. Consequently, in the most contaminated objects, maize plants died out at the seedling stage. In the literature, confirmation can be found of a negative effect of excessive amounts of herbicides on the yield of plants. Baćmaga et al. (2014a) studying the effect of metazachlor on the yield of spring rape demonstrated an unfavourable effect of the preparation on plants. Wyszkowska and Kucharski (2004) also reported a decrease in the fresh mass of spring rape and white mustard under the effect of excessive amounts of the herbicide Triflurotox 250 EC. Similar results were obtained by Baćmaga et al. (2014b) in an experiment using a mixture of diflufenican + mesosulphuron-methyl + iodosulphuron-methyl-sodium. They observed a decrease in the yield of spring wheat, and the application of the two highest doses (18.24 and 36.48 mg kg−1 of DM of soil) resulted in complete dying out of plants. The pesticide Sevin (carbaryl) applied in a dose of 5 dm3 ha−1 also had a negative effect on carrot growth (Elbashier et al. 2016).
Summary
Herbicides may be toxic not only to the target organisms, but also to non-targeted organisms. When entering soil ecosystems, herbicides become a threat to all organisms. These studies describe the effect of a mixture of two active substances (pethoxamid and terbuthylazine) contained in the herbicide Successor T 550 SE on soil microorganisms, soil enzymes and plants. Excessive amounts of this herbicide disturb the biological equilibrium of soil, by disturbing the soil microbiological and biochemical properties. They shift a succession of organotrophic bacteria from the r-strategy to the K-strategy. They also have a negative effect on bacteria of the genus Azotobacter, oligotrophic sporulating bacteria, actinomycetes and fungi and a positive effect on oligotrophic bacteria. A mixture of pethoxamid and terbuthylazine in doses higher than 0.73 mg kg−1 of soil resulted in a strong inhibition of dehydrogenases, catalases, ureases, acid phosphatase, alkaline phosphatase, arylsulphatase and β-glucosidase and significantly inhibited the growth and development of maize.
References
Araujo ASF, Monteiro RTR, Abarkeli RB (2003) Effect of glyphosate on the microbial activity of two Brazilian soil. Chemosphere 52:799–804
Arias-Estévez M, López-Periago E, Martínez-Carballo E, Simal-Gándara J, Mejuto JC, García-Río L (2008) The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric Ecosyst Environ 123(4):247–260
Baćmaga M, Kucharski J, Wyszkowska J, Borowika A, Tomkiel M (2014a) Response of microorganisms and enzymes to soil contamination with metazachlor. Environ Earth Sci 72:2251–2262
Baćmaga M, Wyszkowska J, Borowik A, Tomkiel M, Kucharski J (2014b) Response of Fungi, β-glucosidase and arylsulfatase to Soil Contamination by Alister Grande 190 OD, Fuego 500 SC and Lumax 537,5 SE Herbicides. Pol J Environ Stud 23(1):19–25
Baćmaga M, Borowik A, Kucharski J, Tomkiel M, Wyszkowska J (2015) Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican + mesosulfuron-methyl + iodosulfuron-methyl-sodium. Environ Sci Pollut Res 22:643–656
Caracciolo AB, Giuliano G, Grenni P, Cremisini C, Ciccoli R, Ubaldi C (2005) Effect of urea on degradation of terbuthylazine in soil. Environ Toxicol Chem 24(5):1035–1040
Crouzet O, Batisson I, Besse-Hoggan P, Bonnemoy F, Bardot C, Poly F, Bohatier J, Mallet C (2010) Response of soil microbial communities to the herbicide mesotrione: a dose-effect microcosm approach. Soil Biol Biochem 42:193–202
De Leij FA, Whips JM, Lynch JM (1993) The use of colony development for the characterization of bacterial communities in soil and on roots. Microb Ecol 27:81–97
Dousset S, Mouvet C, Schiavon M (1997) Degradation of [14C] terbuthylazine and [14C] atrazine in laboratory soil microcosms. Pesticid Sci 49(1):9–16
Elbashier MMA, Shao XM, Mohmmed A, Ali AAS, Osman BH (2016) Effect of Pesticide Residues (Sevin) on Carrot (Daucus carota L.) and Free Nitrogen Fixers (Azotobacter spp.). Agric Sci 7:93–99
Floch C, Chevremont AC, Joanico Capowiez Y, Criquet S (2011) Indicators of pesticide contamination: soil enzyme compared to functional diversity of bacterial communities via Biolog® Ecoplates. Eur J Soil Biol 47(4):256–263
FOCUS Soil Modeling Working Group (1997) Soil Persistence models and EU registration. DG VI. European Commission, Doc. 7617/VI/96, Brussels, p 74
HuiXia P, ZhengMing C, XueMei Z, ShuYong M, XiaoLing Q, Fang W (2007) A study on an oligotrophic bacteria and its ecological characteristics in an arid desert area. Sci China Ser D-Earth Sci 50(1):128–134
James TK, Rahman A, Holland PT, McNaughton DE, Heiermann M (1998) Degradation and movement of terbuthylazine in soil. In: Proceedings of the New Zealand Plant Protection Conference. New Zealand Plant Protection Society Inc, pp 157–161
Jastrzębska E, Kucharski J (2007) Dehydrogenases, urease and phosphatases activities of soil contaminated with fungicides. Plant Soil Environ 53(2):51–57
Jezierska-Tys S, Rutkowska A (2013) Soil response to chemicals used in a field experiment. Int Agrophys 27:151–158
Jurina T, Terzić S, Ahel M, Stipičević S, Kontrec D, Kurtanjek Ž, Udiković-Kolić N (2014) Catabolism of terbuthylazine by mixed bacterial culture originating from s-triazine-contaminated soil. Appl Microbiol Biotechnol 98(16):7223–7232
Jursík M, Kočárek M, Hamouzová K, Soukup J, Venclová V (2013) Effect of precipitation on the dissipation, efficacy and selectivity of three chloroacetamide herbicides in sunflower. Plant Soil Environ 59(4):175–182
Jyot G, Mandal K, Singh B (2015) Effect of dehydrogenase, phosphatase and urease activity in cotton soil after applying thiamethoxam as seed treatment. Environ Aonit Assess 187(5):1–7
Kalia A, Gosal SK (2011) Effect of pesticide application on soil microorganisms. Arch Agron Soil Sci 57(6):569–596
Kato S, Kitajima T, Okamoto H, Kobutani T (2001) Pethoxamid-a novel selective herbicide for maize and soybean. In: The BCPC Conference: Weeds, 2001, Proceedings of an international conference held at the Brighton Hilton Metropole Hotel, Brighton, UK, 12–15 Nov 2001, Vol 1 and 2. British Crop Protection Council, pp 23–28
Kucharski J, Tomkiel M, Baćmaga M, Borowik A, Wyszkowska J (2016) Enzyme activity and microorganisms diversity in soil contaminated with the herbicide Boreal 58 WG. J Environ Sci Health B 51(7):446–454
Lo CC (2010) Effect of pesticides on soil microbial community. J Environ Sci Health Part B 45(5):348–359
Lone AH, Raverkar KP, Pareek NAVNEET (2014) In-vitro effects of herbicides on soil microbial communities. Bioscan 9(1):11–16
Martinez CO, Silva CMMS, Fay EF, Maia AHN, Abakerli RB, Durrant LR (2008) Degradation of the herbicide sulfentrazone in a Brazilian Typic Haploudox soil. Soil Biol Biochem 40:879–886
Milošević N, Govedarica M, Cvijanović G (2004) Microorganisms as indicators of herbicide effect on biological activity of soil. Acta Herbol 13(1):243–250
Muñoz-Leoz B, Garbisu C, Antigüedad I, Ruiz-Romera E (2012) Fertilization can modify the non-targed effects of pesticides on soil microbial communities. Soil Biol Biochem 48:125–134
Ratcliff AW, Busse MD, Shestak KJ (2006) Changes in microbial community structure following herbicide (glyphosate) additions to forest soils. Appl Soil Ecol 34:114–124
Riah W, Laval K, Laroche-Ajzenberg E, Mougin C, Latour X, Trinsoutrot-Gattin I (2014) Effects of pesticides on soil enzymes: a review. Environ Chem Lett 12(2):257–273
Saha S, Dutta D, Karmakar R, Prasad Ray D (2012) Structure–toxicity relationship of chloroacetanilide herbicides: relative impact on soil microorganisms. Environ Toxicol Pharmacol 34:307–314
Sahid IB, Teoh SS (1994) Persistence of terbuthylazine in soils. Bull Environ Contam Toxicol 52(2):226–230
Sarathachandra SU, Burch G, Cox NR (1997) Growth patterns of bacterial communities in the rhizoplane and rhizosphere of white clover (Trifolium repens L.) And perennial ryegrass (Lolium perenne L.) In long-term pasture. Appl Soil Ecol 6:293–299
Sebiomo A, Ogundero WV, Bankole AS (2011) Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr J Biotechnol 10(5):770–778
Skrzypczak GA, Pudełko JA, Waniorek W (2007) Assesment of the tank mixture of mesotrione and pethoxamid plus terbuthylazine efficacy for weed control in maize (Zea mays L.). J Plant Prot Res 47(4):375–381
Statsoft, Inc, Statistica (2016) Data Analysis Software System, version 12.5 http://www.statsoft.com.
Tomkiel M, Wyszkowska J, Kucharski J, Baćmaga M, Borowik A (2014) Response of microorganisms and enzymes to soil contamination with the herbicide Successor T 550 SE. Environ Protect Eng 40(4):15–27
Tomkiel M, Baćmaga M, Wyszkowska J, Kucharski J, Borowik A (2015) The effect of carfentrazone-ethyl on soil microorganisms and soil enzymes activity. Arch Environ Prot 41(3):3–10
Vlad CD, Filimon NM, Popescu R, Dumitrascu V, Gurban C, Verds D (2012) Sulfonylureic herbicide influence on bacterial communities in soil. Ann Rom Soc Cell Biol 17(2):77–81
World Reference Base for Soil Resources (2014) A framework for international classification, correlation and communication. World Soils Resources Report. 103, FAO, Rome
Wyszkowska J (2002) Effect of soil contamination with Treflan 480 EC on biochemical properties of soil. Pol J Environ Stud 11(1):71–77
Wyszkowska J, Kucharski J (2004) Biochemical and physicochemical properties of soil contaminated with herbicide Triflurotox 250 EC. Pol J Environ Stud 13(2):223–231
Yao X, Min H, Lü Z, Yuan H (2006) Influence of acetamipirid on soil enzymatic activities and respiration. Eur J Soil Biol 42:120–126
Acknowledgments
These studies were conducted as a part of a research project funded by the National Science Centre, No. N305 386138, and the publication was prepared as a part of statutory activities funded by the Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wyszkowska, J., Tomkiel, M., Baćmaga, M. et al. Response of microorganisms and enzymes to soil contamination with a mixture of pethoxamid terbuthylazine. Environ Earth Sci 75, 1285 (2016). https://doi.org/10.1007/s12665-016-6092-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6092-5