Abstract
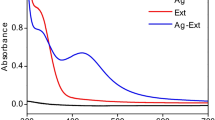
Biosynthesis of silver nanoconjugates using agro-waste has drawn the attention of the researchers in recent years due to eco-friendly and low-cost methods. A comparative study on various techniques (sunlight, probe ultrasonication, and microwave irradiation) of silver-bio-nanoconjugate production was carried out using lyophilized pomegranate peel polyphenols. Polyphenols from pomegranate peel extract were identified by LC–MS. The reaction time of each technique for the synthesis of pomegranate peel’s polyphenols-based silver nanoconjugates was optimized by UV–visible spectroscopy, dynamic light scattering, and antioxidant activity. Further, they were characterized by FT-IR spectroscopy, XRD, FESEM–EDX, elemental mapping, TEM, and zeta potential. UV-spectroscopy and DLS results showed that the sunlight, probe ultrasonication, and microwave irradiation technique could reduce silver (Ag+) ions to silver nanoparticles (Ag0) at λ max ~ 420 nm with Z-average diameters 51.63–94.76, 40.51–61.47, and 43.40–66.52 nm, respectively. The antioxidant activity was maximum at 50 s of microwave irradiation treatment followed by 15 min of probe ultrasonication and 20 min of sunlight exposer. However, TEM analysis revealed that the probe ultrasonication treated nanoconjugates were spherical, smaller, and less aggregated. FT-IR spectra confirmed the involvement and conjugation of various functional groups from pomegranate peel polyphenols. Probe ultrasonic-assisted-silver nanoconjugates showed good antibacterial activity against S. aureus and E. coli as compared to microwave-assisted silver nanoconjugate.
Graphic Abstract













Similar content being viewed by others
Abbreviations
- AA:
-
Antioxidant activity
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- EDX:
-
Energy dispersive X-ray
- FESEM:
-
Field emission scanning electron microscope
- FTIR:
-
Fourier-transform infrared
- HHDP:
-
Hexahydroxydiphenoyl
- LCMS:
-
Liquid chromatography–mass spectroscopy
- PPP:
-
Pomegranate peel polyphenol
- PPP-SNC:
-
Pomegranate peel polyphenol-based silver nanoconjugate
- TE:
-
Total ellagitannin
- TEM:
-
Transmission electron microscope
- TPC:
-
Total phenolic content
- UV–vis:
-
Ultraviolet–visible
- XRD:
-
X-ray diffractometer
- ZOI:
-
Zone of inhibition
References
Nowack, B., Krug, H.F., Height, M.: 120 years of nanosilver history: implications for policy makers (2011). https://doi.org/10.1021/es103316q
Mittal, V.: Polymer layered silicate nanocomposites: a review. Materials 2(3), 992–1057 (2009). https://doi.org/10.3390/ma2030992
Huang, Y., Mei, L., Chen, X., Wang, Q.: Recent developments in food packaging based on nanomaterials. Nanomaterials 8(10), 830 (2018). https://doi.org/10.3390/nano8100830
Sreeram, K.J., Nidhin, M., Nair, B.U.: Microwave assisted template synthesis of silver nanoparticles. Bull. Mater. Sci. 31(7), 937–942 (2008). https://doi.org/10.1007/s12034-008-0149-3
Goharshadi, E.K., Azizi-Toupkanloo, H.: Silver colloid nanoparticles: ultrasound-assisted synthesis, electrical and rheological properties. Powder Technol. 237, 97–101 (2013). https://doi.org/10.1016/j.powtec.2012.12.059
Lu, Z., Meng, M., Jiang, Y., Xie, J.: UV-assisted in situ synthesis of silver nanoparticles on silk fibers for antibacterial applications. Colloids Surf. A 447, 1–7 (2014). https://doi.org/10.1016/j.colsurfa.2014.01.064
Guzmán, M.G., Dille, J., Godet, S.: Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Biomol. Eng. 2(3), 104–111 (2009)
Miao, W., Chan, T.H.: Ionic-liquid-supported synthesis: a novel liquid-phase strategy for organic synthesis. Acc. Chem. Res. 39(12), 897–908 (2006). https://doi.org/10.1021/ar030252f
Wang, S., Zhang, Y., Ma, H.L., Zhang, Q., Xu, W., Peng, J., Zhai, M.: Ionic-liquid-assisted facile synthesis of silver nanoparticle-reduced graphene oxide hybrids by gamma irradiation. Carbon 55, 245–252 (2013). https://doi.org/10.1016/j.carbon.2012.12.033
Aygün, A., Gülbağça, F., Nas, M.S., Alma, M.H., Çalımlı, M.H., Ustaoglu, B., Şen, F.: Biological synthesis of silver nanoparticles using Rheum ribes and evaluation of their anticarcinogenic and antimicrobial potential: a novel approach in phytonanotechnology. J. Pharm. Biomed. Anal. 179, 113012 (2020). https://doi.org/10.1016/j.jpba.2019.113012
Gomathi, A.C., Rajarathinam, S.X., Sadiq, A.M., Rajeshkumar, S.: Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug Deliv. Sci. Technol. 55, 101376 (2020). https://doi.org/10.1016/j.jddst.2019.101376
Madivoli, E.S., Kareru, P.G., Gachanja, A.N., Mugo, S.M., Makhanu, D.S., Wanakai, S.I., Gavamukulya, Y.: Facile synthesis of silver nanoparticles using Lantana trifolia aqueous extracts and their antibacterial activity. J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-019-01432-5
Ottoni, C.A., Lima Neto, M.C., Léo, P., Ortolan, B.D., Barbieri, E., De Souza, A.O.: Environmental impact of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere 239, 124698 (2020). https://doi.org/10.1016/j.chemosphere.2019.124698
Hasnain, M.S., Javed, M.N., Alam, M.S., Rishishwar, P., Rishishwar, S., Ali, S., Beg, S.: Purple heart plant leaves extract-mediated silver nanoparticle synthesis: optimization by Box–Behnken design. Mater. Sci. Eng. C 99, 1105–1114 (2019). https://doi.org/10.1016/j.msec.2019.02.061
Rautela, A., Rani, J., Das, M.D.: Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 10(1), 1–10 (2019). https://doi.org/10.1186/s40543-018-0163-z
Sowmiya, K., Prakash, J.T.J.: Green-synthesis of silver nanoparticles using Abies webbiana LEAVES and evaluation of its antibacterial activity. J. Pharmacogn. Phytochem. 7(5), 2033–2036 (2018)
Shu, M., He, F., Li, Z., Zhu, X., Ma, Y., Zhou, Z., Yang, Z., Gao, F., Zeng, M.: Biosynthesis and antibacterial activity of silver nanoparticles using yeast extract as reducing and capping agents. Nanoscale Res. Lett. 15(1), 14 (2020). https://doi.org/10.1186/s11671-019-3244-z
Foujdar, R., Bera, M.B., Chopra, H.K.: Phenolic nanoconjugates and its application in food. In: Biopolymer-Based Formulations, pp. 751–780. Elsevier (2020). https://doi.org/10.1016/B978-0-12-816897-4.00030-8
Jahan, I., Erci, F., Isildak, I.: Microwave-assisted green synthesis of non-cytotoxic silver nanoparticles using the aqueous extract of Rosa santana (rose) petals and their antimicrobial activity. Anal. Lett. 52(12), 1860–1873 (2019). https://doi.org/10.1080/00032719.2019.1572179
Al-Nuairi, A.G., Mosa, K.A., Mohammad, M.G., El-Keblawy, A., Soliman, S., Alawadhi, H.: Biosynthesis, characterization, and evaluation of the cytotoxic effects of biologically synthesized silver nanoparticles from Cyperus conglomeratus root extracts on breast cancer cell line MCF-7. Biol. Trace Elem. Res. 194(2), 560–569 (2020). https://doi.org/10.1007/s12011-019-01791-7
Khorrami, S., Zarrabi, A., Khaleghi, M., Danaei, M., Mozafari, M.R.: Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 13, 8013 (2018). https://doi.org/10.2147/IJN.S189295
Senthil, B., Devasena, T., Prakash, B., Rajasekar, A.: Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B 177, 1–7 (2017). https://doi.org/10.1016/j.jphotobiol.2017.10.010
Nasr, H.A., Nassar, O.M., El-Sayed, M.H., Kobisi, A.A.: Characterization and antimicrobial activity of lemon peel mediated green synthesis of silver nanoparticles. Int. J. Biol. Chem. 12(2), 56–63 (2020). https://doi.org/10.26577/ijbch-2019-i2-7
Soto, K.M., Quezada-Cervantes, C.T., Hernández-Iturriaga, M., Luna-Bárcenas, G., Vazquez-Duhalt, R., Mendoza, S.: Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT 103, 293–300 (2019). https://doi.org/10.1016/j.lwt.2019.01.023
Kaviya, S., Santhanalakshmi, J., Viswanathan, B., Muthumary, J., Srinivasan, K.: Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 79(3), 594–598 (2011). https://doi.org/10.1016/j.saa.2011.03.040
Tanase, C., Berta, L., Coman, N.A., Roşca, I., Man, A., Toma, F., Mare, A.: Investigation of in vitro antioxidant and antibacterial potential of silver nanoparticles obtained by biosynthesis using beech bark extract. Antioxidants 8(10), 459 (2019). https://doi.org/10.3390/antiox8100459
Burlacu, E., Tanase, C., Coman, N.A., Berta, L.: A review of bark-extract-mediated green synthesis of metallic nanoparticles and their applications. Molecules 24(23), 4354 (2019). https://doi.org/10.3390/molecules24234354
Foujdar, R., Bera, M.B., Chopra, H.K.: Optimization of process variables of probe ultrasonic‐assisted extraction of phenolic compounds from the peel of Punica granatum Var. Bhagwa and it’s chemical and bioactivity characterization. J. Food Process. Preserv. 44(1), 14317 (2020). https://doi.org/10.1111/jfpp.14317
Ben-Ali, S., Akermi, A., Mabrouk, M., Ouederni, A.: Optimization of extraction process and chemical characterization of pomegranate peel extract. Chem. Pap. 72(8), 2087–2100 (2018)
Ravichandran, V., Vasanthi, S., Shalini, S., Shah, S.A.A., Harish, R.: Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater. Lett. 180, 264–267 (2016). https://doi.org/10.1016/j.matlet.2016.05.172
Nasiriboroumand, M., Montazer, M., Barani, H.: Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J. Photochem. Photobiol. B 179, 98–104 (2018). https://doi.org/10.1016/j.jphotobiol.2018.01.006
Shanmugavadivu, M., Kuppusamy, S., Ranjithkumar, R.: Synthesis of pomegranate peel extract mediated silver nanoparticles and its antibacterial activity. Am. J. Adv. Drug Deliv. 2(2), 174–182 (2014)
Deshmukh, A.R., Gupta, A., Kim, B.S.: Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activities. Biomed. Res. Int. (2019). https://doi.org/10.1155/2019/171435
Jayapriya, M., Dhanasekaran, D., Arulmozhi, M., Nandhakumar, E., Senthilkumar, N., Sureshkumar, K.: Green synthesis of silver nanoparticles using Piper longum catkin extract irradiated by sunlight: antibacterial and catalytic activity. Res. Chem. Intermed. 45(6), 3617–3631 (2019). https://doi.org/10.1007/s11164-019-03812-5
Seku, K., Gangapuram, B.R., Pejjai, B., Kadimpati, K.K., Golla, N.: Microwave-assisted synthesis of silver nanoparticles and their application in catalytic, antibacterial and antioxidant activities. J. Nanostruct. Chem. 8(2), 179–188 (2018). https://doi.org/10.1007/s40097-018-0264-7
De Matteis, V., Rizzello, L., Ingrosso, C., Liatsi-Douvitsa, E., De Giorgi, M.L., De Matteis, G., Rinaldi, R.: Cultivar-dependent anticancer and antibacterial properties of silver nanoparticles synthesized using leaves of different olea europaea trees. Nanomaterials 9(11), 1544 (2019). https://doi.org/10.3390/nano9111544
El-Batal, A.I., Gharib, F.A.E.L., Ghazi, S.M., Hegazi, A.Z., Hafz, A.G.M.A.E.: Physiological responses of two varieties of common bean (Phaseolus vulgaris L.) to foliar application of silver nanoparticles. Nanomater. Nanotechnol. 6, 13 (2016). https://doi.org/10.5772/62202
Seeram, N., Lee, R., Hardy, M., Heber, D.: Rapid large-scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 41(1), 49–55 (2005). https://doi.org/10.1016/j.seppur.2004.04.003
Yisimayili, Z., Abdulla, R., Tian, Q., Wang, Y., Chen, M., Sun, Z., Huang, C.: A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 1604, 460472 (2019). https://doi.org/10.1016/j.chroma.2019.460472
Fischer, U.A., Carle, R., Kammerer, D.R.: Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 127(2), 807–821 (2011). https://doi.org/10.1016/j.foodchem.2010.12.156
Mena, P., Calani, L., Dall’Asta, C., Galaverna, G., García-Viguera, C., Bruni, R., Del Rio, D.: Rapid and comprehensive evaluation of (poly) phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 17(12), 14821–14840 (2012). https://doi.org/10.3390/molecules171214821
Preethi, P., Padma, P.R.: Biocompatibility of biosynthesized silver nano bio conjugates derived from a methanolic extract of Piper betle leaves. Indo Am. J. Pharm. Res. 6, 5661–5670 (2016)
Kazemi, M., Karim, R., Mirhosseini, H., Hamid, A.A.: Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: punicalagin and hydroxybenzoic acids. Food Chem. 206, 156–166 (2016). https://doi.org/10.1016/j.foodchem.2016.03.017
Mahajan, P., Sharma, A., Kaur, B., Goyal, N., Gautam, S.: Green synthesized (Ocimum sanctum and Allium sativum) Ag-doped cobalt ferrite nanoparticles for antibacterial application. Vacuum 161, 389–397 (2019)
Pawar, J.S., Patil, R.H.: Green synthesis of silver nanoparticles using Eulophia herbacea (Lindl.) tuber extract and evaluation of its biological and catalytic activity. SN Appl. Sci. 2(1), 52 (2020). https://doi.org/10.1007/s42452-019-1846-9
Baidukova, O., Skorb, E.V.: Ultrasound-assisted synthesis of magnesium hydroxide nanoparticles from magnesium. Ultrason. Sonochem. 31, 423–428 (2016). https://doi.org/10.1016/j.ultsonch.2016.01.034
Khan, M.J., Kumari, S., Shameli, K., Selamat, J., Sazili, A.Q.: Green synthesis and characterization of pullulan mediated silver nanoparticles through ultraviolet irradiation. Materials 12(15), 2382 (2019). https://doi.org/10.3390/ma12152382
Khan, M.J., Shameli, K., Sazili, A.Q., Selamat, J., Kumari, S.: Rapid green synthesis and characterization of silver nanoparticles arbitrated by curcumin in an alkaline medium. Molecules 24(4), 719 (2019). https://doi.org/10.3390/molecules24040719
Sana, S.S., Dogiparthi, L.K.: Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater. Lett. 226, 47–51 (2018). https://doi.org/10.1016/j.matlet.2018.05.009
Fatimah, I.: Green synthesis of silver nanoparticles using extract of Parkia speciosa Hassk pods assisted by microwave irradiation. J. Adv. Res. 7(6), 961–969 (2016). https://doi.org/10.1016/j.jare.2016.10.002
Fatimah, I., Indriani, N.: Silver nanoparticles synthesized using Lantana camara flower extract by reflux, microwave and ultrasound methods. Chem. J. Moldova 13(1), 95–102 (2018). https://doi.org/10.19261/cjm.2017.461
Park, M., Sohn, Y., Shin, W.G., Lee, J., Ko, S.H.: Ultrasonication assisted production of silver nanowires with low aspect ratio and their optical properties. Ultrason. Sonochem. 22, 35–40 (2015). https://doi.org/10.1016/j.ultsonch.2014.05.007
Yin, H., Yamamoto, T., Wada, Y., Yanagida, S.: Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Mater. Chem. Phys. 83(1), 66–70 (2004). https://doi.org/10.1016/j.matchemphys.2003.09.006
Kazemzadeh, S.M., Hassanjani-Roshan, A., Vaezi, M.R., Shokuhfar, A.: The effect of microwave irradiation time on appearance properties of silver nanoparticles. Trans. Indian Inst. Met. 64(3), 261–264 (2011). https://doi.org/10.1007/s12666-011-0053-1
Kumar, B., Smita, K., Cumbal, L., Debut, A.: Ficus carica (Fig) fruit mediated green synthesis of silver nanoparticles and its antioxidant activity: a comparison of thermal and probe ultrasonication approach. BioNanoScience 6(1), 15–21 (2016). https://doi.org/10.1007/s12668-016-0193-1
Ma, G.Z., Wang, C.M., Li, L., Ding, N., Gao, X.L.: Effect of pomegranate peel polyphenols on human prostate cancer PC-3 cells in vivo. Food Sci. Biotechnol. 24(5), 1887–1892 (2015). https://doi.org/10.1007/s10068-015-0247-0
Mahendran, G., Kumari, B.R.: Biological activities of silver nanoparticles from Nothapodytes nimmoniana (Graham) Mabb. fruit extracts. Food Sci. Hum. Wellness 5(4), 207–218 (2016). https://doi.org/10.1016/j.fshw.2016.10.001
Dipankar, C., Murugan, S.: The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B 98, 112–119 (2012). https://doi.org/10.1016/j.colsurfb.2012.04.006
Mittal, A.K., Bhaumik, J., Kumar, S., Banerjee, U.C.: Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 415, 39–47 (2014). https://doi.org/10.1016/j.jcis.2013.10.018
Reddy, N.J., Vali, D.N., Rani, M., Rani, S.S.: Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C 34, 115–122 (2014). https://doi.org/10.1016/j.msec.2013.08.039
Alsamhary, K., Al-Enazi, N., Alshehri, W.A., Ameen, F.: Gold nanoparticles synthesised by flavonoid tricetin as a potential antibacterial nanomedicine to treat respiratory infections causing opportunistic bacterial pathogens. Microb. Pathog. 139, 103928 (2020). https://doi.org/10.1016/j.micpath.2019.103928
Bakhshandeh, R., Shafiekhani, A.: Ultrasonic waves and temperature effects on graphene structure fabricated by electrochemical exfoliation method. Mater. Chem. Phys. 212, 95–102 (2018). https://doi.org/10.1016/j.matchemphys.2018.03.004
Klinkaewnarong, J., Utara, S.: Ultrasonic-assisted conversion of limestone into needle-like hydroxyapatite nanoparticles. Ultrason. Sonochem. 46, 18–25 (2018). https://doi.org/10.1016/j.ultsonch.2018.04.002
Nikolaev, A.L., Gopin, A.V., Severin, A.V., Rudin, V.N., Mironov, M.A., Dezhkunov, N.V.: Ultrasonic synthesis of hydroxyapatite in non-cavitation and cavitation modes. Ultrason. Sonochem. 44, 390–397 (2018). https://doi.org/10.1016/j.ultsonch.2018.02.047
Gopinath, V., MubarakAli, D., Priyadarshini, S., Priyadharsshini, N.M., Thajuddin, N., Velusamy, P.: Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf. B 96, 69–74 (2012). https://doi.org/10.1016/j.colsurfb.2012.03.023
Shrivastava, S., Bera, T., Roy, A., Singh, G., Ramachandrarao, P., Dash, D.: Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18(22), 225103 (2007). https://doi.org/10.1088/0957-4484/18/22/225103
Girón-Vázquez, N.G., Gómez-Gutiérrez, C.M., Soto-Robles, C.A., Nava, O., Lugo-Medina, E., Castrejón-Sánchez, V.H., Luque, P.A.: Study of the effect of Persea americana seed in the green synthesis of silver nanoparticles and their antimicrobial properties. Results Phys. 13, 102142 (2019). https://doi.org/10.1016/j.rinp.2019.02.078
López-Esparza, J., Espinosa-Cristóbal, L.F., Donohue-Cornejo, A., Reyes-López, S.Y.: Antimicrobial activity of silver nanoparticles in polycaprolactone nanofibers against gram-positive and gram-negative bacteria. Ind. Eng. Chem. Res. 55(49), 12532–12538 (2016). https://doi.org/10.1021/acs.iecr.6b02300
Arokiyaraj, S., Saravanan, M., Prakash, N.U., Arasu, M.V., Vijayakumar, B., Vincent, S.: Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: an in vitro study. Mater. Res. Bull. 48(9), 3323–3327 (2013). https://doi.org/10.1016/j.materresbull.2013.05.059
Madubuonu, N., Aisida, S.O., Ahmad, I., Botha, S., Zhao, T.K., Maaza, M., Ezema, F.I.: Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A 126(1), 1–8 (2020). https://doi.org/10.1007/s00339-019-3249-6
Zafar, S., Ashraf, A., Ijaz, M.U., Muzammil, S., Siddique, M.H., Afzal, S., Mahboob, S.: Eco-friendly synthesis of antibacterial zinc nanoparticles using Sesamum indicum L. extract. J. King Saud Univ. Sci. 32(1), 1116–1122 (2020). https://doi.org/10.1016/j.jksus.2019.10.017
Chandra, H., Patel, D., Kumari, P., Jangwan, J.S., Yadav, S.: Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 102, 212–220 (2019). https://doi.org/10.1016/j.msec.2019.04.035
Sriramulu, M., Shanmugam, S., Ponnusamy, V.K.: Agaricus bisporus mediated biosynthesis of copper nanoparticles and its biological effects: an in-vitro study. Colloid Interface Sci. Commun. 35, 100254 (2020). https://doi.org/10.1016/j.colcom.2020.100254
Chaudhary, J., Tailor, G., Yadav, B.L., Michael, O.: Synthesis and biological function of Nickel and Copper nanoparticles. Heliyon 5(6), e01878 (2019). https://doi.org/10.1016/j.heliyon.2019.e01878
Salari, S., Bahabadi, S.E., Samzadeh-Kermani, A., Yosefzaei, F.: In-vitro evaluation of antioxidant and antibacterial potential of greensynthesized silver nanoparticles using Prosopis farcta fruit extract. Iran. J. Pharm. Res. IJPR 18(1), 430 (2019). https://www.ncbi.nlm.nih.gov/pmc/articles/pmc6487442/
Acknowledgements
Financial support in the form of Institute fellowship from MHRD, New Delhi, India, technical assistance provided by the laboratory of sophisticated analytical instrumentation facility, Panjab University, Chandigarh and central laboratory of Punjab Agriculture University, Ludhiana for providing the facility of FESEM, LC-MS and TEM image analysis is gratefully acknowledged.
Funding
Funding was provided by Ministry of Human Resource Development (Grant No. Dean(A)/Ph. D/8047).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest for this research article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Foujdar, R., Chopra, H.K., Bera, M.B. et al. Effect of Probe Ultrasonication, Microwave and Sunlight on Biosynthesis, Bioactivity and Structural Morphology of Punica granatum Peel’s Polyphenols-Based Silver Nanoconjugates. Waste Biomass Valor 12, 2283–2302 (2021). https://doi.org/10.1007/s12649-020-01175-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01175-2