Abstract
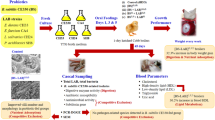
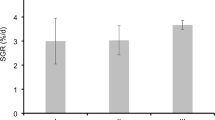
A potential host-derived probiotic, Bacillus subtilis 6-3-1, was successfully screened from 768 isolates from the intestines of healthy hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) based on multiple probiotic characteristics in vitro assays, such as, non-hemolytic activity, extracellular enzyme activity, inhibitory activity against pathogens, tolerance to gastrointestinal stress, cell surface hydrophobicity, autoaggregation, and antibiotic susceptibility. Eight weeks of feeding trial revealed that dietary supplementation of B. subtilis 6-3-1 at all three concentrations (1 × 106 CFU g−1 as BS6; 1 × 107 CFU g−1 as BS7; 1 × 108 CFU g−1 as BS8) could promote the growth performance of hybrid groupers to a certain extent at different time points. At the end of 8th week, BS6 and BS8 significantly promoted the weight gain rate (WGR), specific growth rate (SGR) of hybrid groupers. The digestive enzyme activities were also increased in BS6 and BS8 groups comparing with those in control group, except that the increase of amylase activities in BS6 was not significant (P > 0.05). However, BS7 showed the best non-specific immunity stimulating effects among the three concentration groups. While BS7 significantly boosted serum total protein contents, lysozyme (LZM), total antioxidant capacity (T-AOC), superoxide dismutase (SOD), catalase (CAT), and acid phosphatase (ACP) levels, BS6 significantly enhanced serum total protein, LZM activity, and BS8 significantly improved LZM, respiratory bursts activity. B. subtilis 6-3-1 up-regulated the expression of MyD88 in head kidney and intestine and increased villi length (VL) in intestine of BS7 group. It also up-regulated the expression of IgM in head kidney in BS6 group and IgM and TLR1 in intestine of BS8 group. Though all B. subtilis 6-3-1 supplemented groups reduced the cumulative mortality rate post-Vibro harveyi-challenge, BS7 showed the best protection effects among the three concentration groups. In conclusion, with its immune promoting, intestine health enhancing, and V. harveyi resisting effects, BS7 show great potential to be used as a probiotic in hybrid grouper culture.















Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request and its supplementary information file.
References
Sun Y, Guo C-Y, Wang D-D, Li XF, Xiao L, Zhang X, You X, Shi Q, Hu G-J, Fang C, Lin H-R, Zhang Y (2016) Transcriptome analysis reveals the molecular mechanisms underlying growth superiority in a novel grouper hybrid (Epinephelus fuscogutatus ♀ × E. lanceolatus ♂). BMC Genetics 17(1):24. https://doi.org/10.1186/s12863-016-0328-y
Li X (2017) What is the situation of grouper industry in 2017? Here is the market research from the front line (Chinese article). Current Fisheries 42(03):40–42. https://doi.org/10.3969/j.issn.1674-9049.2017.03.008
Harikrishnan R, Balasundaram C, Heo M (2010) Molecular studies, disease status and prophylactic measures in grouper aquaculture: economic importance, diseases and immunology. Aquaculture 309(1):1–14. https://doi.org/10.1016/j.aquaculture.2010.09.011
Li J, Wu Z-B, Zhang Z, Zha J-W, Qu S-Y, Qi X-Z, Wang G-X, Ling F (2019) Effects of potential probiotic Bacillus velezensis K2 on growth, immunity and resistance to Vibrio harveyi infection of hybrid grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀). Fish Shellfish Immun 93:1047–1055. https://doi.org/10.1016/j.fsi.2019.08.047
Liu S, Wang S, Cai Y, Li E, Ren Z, Wu Y, Guo W, Sun Y, Zhou Y (2019) Beneficial effects of a host gut-derived probiotic, Bacillus pumilus, on the growth, non-specific immune response and disease resistance of juvenile golden pompano, Trachinotus ovatus. Aquaculture:734446. https://doi.org/10.1016/j.aquaculture.2019.734446
Loh JY, Chan HK, Yam HC, In LLA, Lim CSY (2020) An overview of the immunomodulatory effects exerted by probiotics and prebiotics in grouper fish. Aquac Int 28(2):729–750. https://doi.org/10.1007/s10499-019-00491-2
Harikrishnan R, Balasundaram C, Heo M-S (2011) Fish health aspects in grouper aquaculture. Aquaculture 320(1):1–21. https://doi.org/10.1016/j.aquaculture.2011.07.022
Lee S, Katya K, Park Y, Won S, Seonghamidoghli A, Bai SC, M (2017) Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immun 61:201–210. https://doi.org/10.1016/j.fsi.2016.12.035
Andani HRR, Tukmechi A, Meshkini S, Sheikhzadeh N (2012) Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 28(5):728–734. https://doi.org/10.1111/j.1439-0426.2012.01974.x
Cai Y, Yuan W, Wang S, Guo W, Li A, Wu Y, Chen X, Ren Z, Zhou Y (2019) In vitro screening of putative probiotics and their dual beneficial effects: to white shrimp (Litopenaeus vannamei) postlarvae and to the rearing water. Aquaculture 498:61–71. https://doi.org/10.1016/j.aquaculture.2018.08.024
Wang A, Ran C, Wang Y, Zhang Z, Ding Q, Yang Y, Olsen RE, Ringø E, Bindelle J, Zhou Z (2019) Use of probiotics in aquaculture of China—a review of the past decade. Fish Shellfish Immun 86:734–755. https://doi.org/10.1016/j.fsi.2018.12.026
Kuebutornye FKA, Abarike ED, Lu Y (2019) A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immun 87:820–828. https://doi.org/10.1016/j.fsi.2019.02.010
Zhou S, Song D, Zhou X, Mao X, Zhou X, Wang S, Wei J, Huang Y, Wang W, Xiao S-M, Qin Q (2019) Characterization of Bacillus subtilis from gastrointestinal tract of hybrid Hulong grouper (Epinephelus fuscoguttatus × E. lanceolatus) and its effects as probiotic additives. Fish Shellfish Immun 84:1115–1124. https://doi.org/10.1016/j.fsi.2018.10.058
Liu C-H, Chiu C-H, Wang S-W, Cheng W (2012) Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immun 33(4):699–706. https://doi.org/10.1016/j.fsi.2012.06.012
Tang L, Huang K, Xie J, Yu D, Sun L, Huang Q, Bi Y (2017) 1-Deoxynojirimycin from Bacillus subtilis improves antioxidant and antibacterial activities of juvenile Yoshitomi tilapia. Electron J Biotechnol 30:39–47. https://doi.org/10.1016/j.ejbt.2017.08.006
Liu H, Wang S, Cai Y, Guo X, Cao Z, Zhang Y, Liu S, Yuan W, Zhu W, Zheng Y, Xie Z, Guo W, Zhou Y (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immun 60:326–333. https://doi.org/10.1016/j.fsi.2016.12.003
Telli GS, Ranzani-Paiva MJT, Dias DdC, Sussel FR, Ishikawa CM, Tachibana L (2014) Dietary administration of Bacillus subtilis on hematology and non-specific immunity of Nile tilapia Oreochromis niloticus raised at different stocking densities. Fish Shellfish Immun 39(2):305–311. https://doi.org/10.1016/j.fsi.2014.05.025
Abarike ED, Cai J, Lu Y, Yu H, Chen L, Jian J, Tang J, Jun L, Kuebutornye FKA (2018) Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immun 82:229–238. https://doi.org/10.1016/j.fsi.2018.08.037
Galagarza OA, Smith SA, Drahos DJ, Eifert JD, Williams RC, Kuhn DD (2018) Modulation of innate immunity in Nile tilapia (Oreochromis niloticus) by dietary supplementation of Bacillus subtilis endospores. Fish Shellfish Immun 83:171–179. https://doi.org/10.1016/j.fsi.2018.08.062
Tang S, Liu S, Zhang J, Zhou L, Wang X, Zhao Q, Weng W, Qin JG, Chen L, Li E (2020) Relief of hypersaline stress in Nile tilapia Oreochromis niloticus by dietary supplementation of a host-derived Bacillus subtilis strain. Aquaculture:735542. https://doi.org/10.1016/j.aquaculture.2020.735542
Zhang Q, Yu H, Tong T, Tong W, Dong L, Xu M, Wang Z (2014) Dietary supplementation of Bacillus subtilis and fructooligosaccharide enhance the growth, non-specific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against Vibrio vulnificus. Fish Shellfish Immun 38(1):7–14. https://doi.org/10.1016/j.fsi.2014.02.008
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Bakr A, El-Keredy AMS, Dawood MAO, Dossou S, Wang W, Yukun Z (2018) Bacillus subtilis as probiotic candidate for red sea bream: growth performance, oxidative status, and immune response traits. Fish Shellfish Immun 79:303–312. https://doi.org/10.1016/j.fsi.2018.05.035
Cerezuela R, Fumanal M, Tapia-Paniagua ST, Meseguer J, Moriñigo MÁ, Esteban MÁ (2013) Changes in intestinal morphology and microbiota caused by dietary administration of inulin and Bacillus subtilis in gilthead sea bream (Sparus aurata L.) specimens. Fish Shellfish Immun 34(5):1063–1070. https://doi.org/10.1016/j.fsi.2013.01.015
Tang Y, Han L, Chen X, Xie M, Kong W, Wu Z (2019) Dietary supplementation of probiotic Bacillus subtilis affects antioxidant defenses and immune response in grass carp under Aeromonas hydrophila challenge. Probiotics Antimicrob Proteins 11(2):545–558. https://doi.org/10.1007/s12602-018-9409-8
Zhang D, Wu Z, Chen X, Wang H, Guo D (2019) Effect of Bacillus subtilis on intestinal apoptosis of grass carp Ctenopharyngodon idella orally challenged with Aeromonas hydrophila. Fish Sci 85(1):187–197. https://doi.org/10.1007/s12562-018-1272-8
Wu ZX, Feng X, Xie LL, Peng XY, Yuan J, Chen XX (2012) Effect of probiotic Bacillus subtilis Ch9 for grass carp, Ctenopharyngodon idella (Valenciennes, 1844), on growth performance, digestive enzyme activities and intestinal microflora. J Appl Ichthyol 28(5):721–727. https://doi.org/10.1111/j.1439-0426.2012.01968.x
Zhao H, Ye L, Zhang Y, Chen X, Wang H, Guo D, Wu Z (2020) Effects of Bacillus subtilis on hepatic lipid metabolism and oxidative stress response in grass carp (Ctenopharyngodon idellus) fed a high-fat diet. Marine Life Science & Technology 2(1):50–59. https://doi.org/10.1007/s42995-019-00005-2
He S, Zhang Y, Xu L, Yang Y, Marubashi T, Zhou Z, Yao B (2013) Effects of dietary Bacillus subtilis C-3102 on the production, intestinal cytokine expression and autochthonous bacteria of hybrid tilapia Oreochromis niloticus ♀×Oreochromis aureus ♂. Aquaculture 412–413:125–130. https://doi.org/10.1016/j.aquaculture.2013.06.028
Guo X, Chen D-D, Peng K-S, Cui Z-W, Zhang X-J, Li S, Zhang Y-A (2016) Identification and characterization of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish Immun 52:74–84. https://doi.org/10.1016/j.fsi.2016.03.017
Cao H, Yu R, Zhang Y, Hu B, Jian S, Wen C, Kajbaf K, Kumar V, Yang G (2019) Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 508:106–112. https://doi.org/10.1016/j.aquaculture.2019.04.064
Liu C-H, Wu K, Chu T-W, Wu T-M (2018) Dietary supplementation of probiotic, Bacillus subtilis E20, enhances the growth performance and disease resistance against Vibrio alginolyticus in parrot fish (Oplegnathus fasciatus). Aquac Int 26(1):63–74. https://doi.org/10.1007/s10499-017-0189-z
Geng X, Dong X-H, Tan B-P, Yang Q-H, Chi S-Y, Liu H-Y, Liu X-Q (2011) Effects of dietary chitosan and Bacillus subtilis on the growth performance, non-specific immunity and disease resistance of cobia Rachycentron canadum. Fish Shellfish Immun 31(3):400–406. https://doi.org/10.1016/j.fsi.2011.06.006
Kumar R, Mukherjee SC, Ranjan R, Vani T, Brahmachari RK, Nayak SK (2015) Effect of dietary supplementation of Bacillus subtilis on haematological and immunological parameters of Catla catla (Hamilton). Aquac Int 23(5):1275–1292. https://doi.org/10.1007/s10499-015-9883-x
Ai Q, Xu H, Mai K, Xu W, Wang J, Zhang W (2011) Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker Larimichthys crocea. Aquaculture 317(1):155–161. https://doi.org/10.1016/j.aquaculture.2011.04.036
Kumar R, Mukherjee SC, Ranjan R, Nayak SK (2008) Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immun 24(2):168–172. https://doi.org/10.1016/j.fsi.2007.10.008
Di J, Chu Z, Zhang S, Huang J, Du H, Wei Q (2019) Evaluation of the potential probiotic Bacillus subtilis isolated from two ancient sturgeons on growth performance, serum immunity and disease resistance of Acipenser dabryanus. Fish Shellfish Immun 93:711–719. https://doi.org/10.1016/j.fsi.2019.08.020
Fan Y, Liu L, Zhao L, Wang X, Wang D, Huang C, Zhang J, Ji C, Ma Q (2018) Influence of Bacillus subtilis ANSB060 on growth, digestive enzyme and aflatoxin residue in Yellow River carp fed diets contaminated with aflatoxin B1. Food Chem Toxicol 113:108–114. https://doi.org/10.1016/j.fct.2018.01.033
Yang HL, Sun YZ, Hu X, Ye JD, Lu KL, Hu LH, Zhang JJ (2019) Bacillus pumilus SE5 originated PG and LTA tuned the intestinal TLRs/MyD88 signaling and microbiota in grouper (Epinephelus coioides). Fish Shellfish Immun 88:266–271. https://doi.org/10.1016/j.fsi.2019.03.005
Sela U, Euler CW, Correa da Rosa J, Fischetti VA (2018) Strains of bacterial species induce a greatly varied acute adaptive immune response: the contribution of the accessory genome. PLoS Pathog 14(1):e1006726. https://doi.org/10.1371/journal.ppat.1006726
Argyri AA, Zoumpopoulou G, Karatzas K-AG, Tsakalidou E, Nychas G-JE, Panagou EZ, Tassou CC (2013) Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol 33(2):282–291. https://doi.org/10.1016/j.fm.2012.10.005
Thankappan B, Ramesh D, Ramkumar S, Natarajaseenivasan K, Anbarasu K (2015) Characterization of Bacillus spp. from the gastrointestinal tract of Labeo rohita—towards to identify novel probiotics against fish pathogens. Appl Biochem Biotechnol 175(1):340–353. https://doi.org/10.1007/s12010-014-1270-y
Charteris WP, Kelly PM, Morelli L, Collins JK (1998) Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 84(5):759–768. https://doi.org/10.1046/j.1365-2672.1998.00407.x
Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E (2006) Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J 16(3):189–199. https://doi.org/10.1016/j.idairyj.2005.02.009
Del Re B, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31(6):438–442. https://doi.org/10.1046/j.1365-2672.2000.00845.x
Patel AK, Ahire JJ, Pawar SP, Chaudhari BL, Chincholkar SB (2009) Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res Int 42(4):505–510. https://doi.org/10.1016/j.foodres.2009.01.013
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882. https://doi.org/10.1093/nar/25.24.4876
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. https://doi.org/10.1093/molbev/msr121
Temmerman R, Pot B, Huys G, Swings J (2003) Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol 81(1):1–10. https://doi.org/10.1016/S0168-1605(02)00162-9
Wei YC, Pan TS, Chang MX, Huang B, Xu Z, Luo TR, Nie P (2011) Cloning and expression of Toll-like receptors 1 and 2 from a teleost fish, the orange-spotted grouper Epinephelus coioides. Vet Immunol Immunopathol 141(3):173–182. https://doi.org/10.1016/j.vetimm.2011.02.016
Cui M, Zhang Q, Yao Z, Zhang Z, Zhang H, Wang Y (2010) Immunoglobulin M gene expression analysis of orange-spotted grouper, Epinephelus coioides, following heat shock and Vibrio alginolyticus challenge. Fish Shellfish Immun 29(6):1060–1065. https://doi.org/10.1016/j.fsi.2010.08.018
Kirubakaran CJW, Subramani PA, Michael RD (2016) Methanol extract of Nyctanthes arbortristis seeds enhances non-specific immune responses and protects Oreochromis mossambicus (Peters) against Aeromonas hydrophila infection. Res Vet Sci 105:243–248. https://doi.org/10.1016/j.rvsc.2016.02.013
Bullerwell CN, Collins SA, Lall SP, Anderson DM (2016) Growth performance, proximate and histological analysis of rainbow trout fed diets containing Camelina sativa seeds, meal (high-oil and solvent-extracted) and oil. Aquaculture 452:342–350. https://doi.org/10.1016/j.aquaculture.2015.11.008
Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immun 25(1):128–136. https://doi.org/10.1016/j.fsi.2008.03.013
Park Y, Lee S, Hong J, Kim D, Moniruzzaman M, Bai SC (2017) Use of probiotics to enhance growth, stimulate immunity and confer disease resistance to Aeromonas salmonicida in rainbow trout (Oncorhynchus mykiss). Aquac Res 48(6):2672–2682. https://doi.org/10.1111/are.13099
Sadat Hoseini Madani N, Adorian TJ, Ghafari Farsani H, Hoseinifar SH (2018) The effects of dietary probiotic Bacilli (Bacillus subtilis and Bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) postlarvae. Aquac Res 49(5):1926–1933. https://doi.org/10.1111/are.13648
Rengpipat S, Rukpratanporn S, Piyatiratitivorakul S, Menasaveta P (2000) Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 191(4):271–288. https://doi.org/10.1016/S0044-8486(00)00440-3
Tseng D-Y, Ho P-L, Huang S-Y, Cheng S-C, Shiu Y-L, Chiu C-S, Liu C-H (2009) Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immun 26(2):339–344. https://doi.org/10.1016/j.fsi.2008.12.003
Zhao Y, Zhang W, Xu W, Mai K, Zhang Y, Liufu Z (2012) Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immun 32(5):750–755. https://doi.org/10.1016/j.fsi.2012.01.027
Xiaolong G, Caihuan K, Mo Z, Xian L, Fucun W, Ying L (2019) Effects of the probiotic Bacillus amyloliquefaciens on the growth, immunity, and disease resistance of Haliotis discus hannai. Fish Shellfish Immun 94:617–627. https://doi.org/10.1016/j.fsi.2019.08.067
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immun 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Lee S, Katya K, Hamidoghli A, Hong J, Kim D-J, Bai SC (2018) Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immun 83:283–291. https://doi.org/10.1016/j.fsi.2018.09.031
Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI (2016) Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immun 57:170–178. https://doi.org/10.1016/j.fsi.2016.08.038
Kusano C, Ferrari B (2008) Total antioxidant capacity: a biomarker in biomedical and nutritional studies. J Cell Mol Biol 7(1):1–15
Deng J, Kang B, Tao L, Rong H, Zhang X (2013) Effects of dietary cholesterol on antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in rainbow trout (Oncorhynchus mykiss) fed soybean meal-based diets. Fish Shellfish Immun 34(1):324–331. https://doi.org/10.1016/j.fsi.2012.11.008
Martínez-Alvarez R, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88. https://doi.org/10.1007/s11160-005-7846-4
Chiu C-H, Cheng C-H, Gua W-R, Guu Y-K, Cheng W (2010) Dietary administration of the probiotic, Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immun 29(6):1053–1059. https://doi.org/10.1016/j.fsi.2010.08.019
Zhou X, Tian Z, Wang Y, Li W (2010) Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem 36(3):501–509. https://doi.org/10.1007/s10695-009-9320-z
Sun Y-Z, Yang H-L, Ma R-L, Lin W-Y (2010) Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immun 29(5):803–809. https://doi.org/10.1016/j.fsi.2010.07.018
Bilal S, Lie KK, Karlsen OA, Hordvik I (2016) Characterization of IgM in Norwegian cleaner fish (lumpfish and wrasses). Fish Shellfish Immun 59:9–17. https://doi.org/10.1016/j.fsi.2016.09.063
Magnadottir B (2010) Immunological control of fish diseases. Mar Biotechnol 12(4):361–379. https://doi.org/10.1007/s10126-010-9279-x
Cuesta A, Meseguer J, Esteban MA (2004) Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.). Vet Immunol Immunopathol 101(3):203–210. https://doi.org/10.1016/j.vetimm.2004.04.021
Akira S, Hemmi H (2003) Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 85(2):85–95. https://doi.org/10.1016/S0165-2478(02)00228-6
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2(8):675–680. https://doi.org/10.1038/90609
Janeway CA, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20(1):197–216. https://doi.org/10.1146/annurev.immunol.20.083001.084359
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406(6797):782–787. https://doi.org/10.1038/35021228
Wang Y, Li J, Han J, Shu C, Xu T (2016) Identification and characteristic analysis of TLR28: a novel member of the TLR1 family in teleost. Dev Comp Immunol 62:102–107. https://doi.org/10.1016/j.dci.2016.05.001
Wu M, Guo L, Zhu K-C, Guo H-Y, Liu B, Jiang S-G, Zhang D-C (2018) Genomic structure and molecular characterization of Toll-like receptors 1 and 2 from golden pompano Trachinotus ovatus (Linnaeus, 1758) and their expression response to three types of pathogen-associated molecular patterns. Dev Comp Immunol 86:34–40. https://doi.org/10.1016/j.dci.2018.04.022
Han P, Wang S, Zhang Q, Zhang S, Shao R, Xu W, Zhang W, Xu Q, Wei Q, Qi Z (2018) Molecular characterization and expression analysis of TLR1 and TLR4 from the endangered fish Dabry’s sturgeon (Acipenser dabryanus). Dev Comp Immunol 86:180–188. https://doi.org/10.1016/j.dci.2018.05.009
de Medina FS, Ortega-González M, González-Pérez R, Capitán-Cañadas F, Martínez-Augustin O (2013) Host–microbe interactions: the difficult yet peaceful coexistence of the microbiota and the intestinal mucosa. Br J Nutr 109(S2):S12–S20. https://doi.org/10.1017/S0007114512004035
Rauta PR, Samanta M, Dash HR, Nayak B, Das S (2014) Toll-like receptors (TLRs) in aquatic animals: signaling pathways, expressions and immune responses. Immunol Lett 158(1):14–24. https://doi.org/10.1016/j.imlet.2013.11.013
Yang HL, Xia HQ, Ye YD, Zou WC, Sun YZ (2014) Probiotic Bacillus pumilus SE5 shapes the intestinal microbiota and mucosal immunity in grouper Epinephelus coioides. Dis Aquat Org 111(2):119–127. https://doi.org/10.3354/dao02772
Lin Y-S, Saputra F, Chen Y-C, Hu S-Y(2018) Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio), Fish Shellfish Immun. https://doi.org/10.1016/j.fsi.2018.11.047
Ren Y, Xue J, Yang H, Pan B, Bu W (2017) Comparative and evolutionary analysis of an adapter molecule MyD88 in invertebrate metazoans. Dev Comp Immunol 76:18–24. https://doi.org/10.1016/j.dci.2017.05.007
Dunne A, Ejdeback M, Ludidi PL, O’Neill LAJ, Gay NJ (2003) Structural complementarity of Toll/interleukin-1 receptor domains in Toll-like receptors and the adaptors Mal and MyD88. J Biol Chem 278(42):41443–41451. https://doi.org/10.1074/jbc.m301742200
Crick F (1970) Central dogma of molecular biology. Nature 227(5258):561–563. https://doi.org/10.1038/227561a0
Del’Duca A, Evangelista Cesar D, Galuppo Diniz C, Abreu PC (2013) Evaluation of the presence and efficiency of potential probiotic bacteria in the gut of tilapia (Oreochromis niloticus) using the fluorescent in situ hybridization technique. Aquaculture 388–391:115–121. https://doi.org/10.1016/j.aquaculture.2013.01.019
Divya M, Gopi N, Iswarya A, Govindarajan M, Alharbi NS, Kadaikunnan S, Khaled JM, Almanaa TN, Vaseeharan B (2020) β-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb Pathog 139:103917. https://doi.org/10.1016/j.micpath.2019.103917
Funding
This study was funded by the National Natural Science Foundation of China (No. 31860739, 42066007), Major science and technology projects in Hainan Province (No. ZDKJ2019011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was approved by the Animal Use and Care Committee of Hainan University (Approval Number: HNUAUCC-2020-00011).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12602_2021_9768_MOESM1_ESM.docx
Supplementary file1 Supplementary Information. The online version contains supplementary material available at website of Probiotics Antimicrob Proteins. (DOCX 225 KB)
Rights and permissions
About this article
Cite this article
Liao, J., Cai, Y., Wang, X. et al. Effects of a Potential Host Gut-Derived Probiotic, Bacillus subtilis 6-3-1, on the Growth, Non-specific Immune Response and Disease Resistance of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Probiotics & Antimicro. Prot. 13, 1119–1137 (2021). https://doi.org/10.1007/s12602-021-09768-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09768-6