Abstract
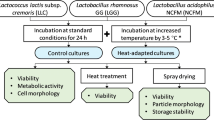
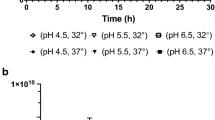
Loss in probiotic viability upon exposure to stressful storage and transport conditions has plagued the probiotic market worldwide. Lactobacillus acidophilus is an important probiotic that is added to various functional foods. It is known to be fairly labile and susceptible to temperature variations that it encounters during processing and storage which increases production cost. It has been repeatedly demonstrated that pre-exposure to sub-lethal doses of stress, particularly, temperature and pH, leads to improved survival of various probiotics when they subsequently encounter the same stress of a much greater magnitude. Attempts to adapt L. acidophilus to temperatures as high as 65 °C to arrive at a thermotolerant variant have not been reported previously. To improve viability at elevated temperatures, we gradually adapted the L. acidophilus NCFM strain to survival at 65 °C for 40 min. Following adaptation, the variant showed a 2-log greater survival compared to wild-type at 65 °C. Interestingly, this thermotolerant variant also demonstrated a 2-log greater stability compared to wild-type at pH 2.0. The improved pH and temperature stress tolerance exhibited by this variant remained unaltered even when the strain was lyophilized. Moreover, the thermotolerant variant demonstrated improved stability compared to wild-type when stored for up to a week at 37 and 42 °C. Probiotic properties of the variant such as adherence to epithelial cells and antibacterial activity remained unaltered. This strain can potentially help address the issue of significant loss in viable cell counts of L. acidophilus which is typically encountered during probiotic manufacture and storage.







Similar content being viewed by others
References
Mills S, Stanton C, Fitzgerald GF, Ross RP (2011) Enhancing the stress responses of probiotics for a lifestyle from gut to product and back again. Microb Cell Factories 10(Suppl 1):S19. https://doi.org/10.1186/1475-2859-10-S1-S19
Mills S, Stanton C, Ross RP (2009) Microbial production of bioactives: from fermented functional foods to probiotic mechanisms. Austral J Dairy Technol 64(1):41–49
O'Flaherty S, Saulnier DM, Pot B, Versalovic J (2010) How can probiotics and prebiotics impact mucosal immunity? Gut Microbes 1(5):293–300. https://doi.org/10.4161/gmic.1.5.12924
Ross RP, Mills S, Hill C, Fitzgerald GF, Stanton C (2010) Specific metabolite production by gut microbiota as a basis for probiotic function. Int Dairy J 20(4):269–276. https://doi.org/10.1016/j.idairyj.2009.12.003
Stack HM, Kearney N, Stanton C, Fitzgerald GF, Ross RP (2010) Association of beta-glucan endogenous production with increased stress tolerance of intestinal lactobacilli. Appl Environ Microbiol 76(2):500–507. https://doi.org/10.1128/AEM.01524-09
Meng XC, Stanton C, Fitzgerald GF, Daly C, Ross RP (2008) Anhydrobiotics: the challenges of drying probiotic cultures. Food Chem 106(4):1406–1416. https://doi.org/10.1016/j.foodchem.2007.04.076
Jennings TA (1999) Lyophilization - introduction and basic principles. CRC press, Boca Raton
Santivarangkna C, Kulozik U, Foerst P (2007) Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol Prog 23(2):302–315. https://doi.org/10.1021/bp060268f
Teixeira P, Castro H, Mohacsi-Farkas C, Kirby R (1997) Identification of sites of injury in Lactobacillus bulgaricus during heat stress. J Appl Microbiol 83(2):219–226. https://doi.org/10.1046/j.1365-2672.1997.00221.x
Somero GN (1995) Proteins and temperature. Annu Rev Physiol 57:43–68. https://doi.org/10.1146/annurev.ph.57.030195.000355
Wang YC, Yu RC, Chou CC (2004) Viability of lactic acid bacteria and bifidobacteria in fermented soymilk after drying, subsequent rehydration and storage. Int J food Microbiol 93(2):209–2017. https://doi.org/10.1016/j.ijfoodmicro.2003.12.001
Gardiner G, O’Sullivan E, Kelly J, Auty MAE, Fitzgerald GF, Collins JK, Ross RP, Stanton C (2000) Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salvarius strains during heat treatment and spray drying. Appl Environ Microbiol 66(6):2605–2616. https://doi.org/10.1128/AEM.66.6.2605-2612.2000
Gueimonde M, Sanchez B (2012) Enhancing probiotic stability in industrial processes. Microbiol Ecol Health Dis 23:18562. https://doi.org/10.3402/mehd.v23i0.18562
Corcoran BM, Stanton C, Fitzgerald GF, Ross RP (2008) Life under stress: the probiotic stress response and how it may be manipulated. Curr Pharm Des 14(14):1382–1399. https://doi.org/10.2174/138161208784480225
Begley M, Gahan CG, Hill C (2005) The interation between bacteria and bile. FEMS Microbiol Rev 29(4):625–651. https://doi.org/10.1016/j.femsre.2004.09.003
van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Magiun E (2002) Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187–216
Corcoran BM, Ross RP, Fitzgerald GF, Dockery P, Stanton C (2006) Enhanced survival of GroESL-overproducing Lactobacillus paracasei NFBC 338 under stressful conditions induced by drying. Appl Environ Microbiol 72(7):5104–5107. https://doi.org/10.1128/AEM.02626-05
De Angelis M, Gobbetti M (2004) Environmental stress responses in Lactobacillus: a review. Proteomics 4(1):106–122. https://doi.org/10.1002/pmic.200300497
Goeusbet G, Jan G, Boyaval P (2002) Two-dimensional electrophoresis study of Lactobacillus delbreuckii subsp. bulgaricus thermotolerance. Appl Environ Microbiol 68(3):1055–1063. https://doi.org/10.1128/AEM.68.3.1055-1063.2002
Ricciardi A, Parente E, Guidone A, Ianniello RG, Zotta T, Abu Sayem SM, Varcamonti M (2012) Genotypic diversity of stress response in Lactobacillus plantarum, Lactobacillus paraplantarum and Lactobacillus pentosus. Int J Food Microbiol 157(2):278–285. https://doi.org/10.1016/j.ijfoodmicro.2012.05.018
Flahaut S, Frere J, Boutibonnes P, Auffray Y (1997) Relationship between the thermotolerance and increase of DnaK and GroEL synthesis in Enterococcus faecalis ATCC19433. J Basic Microbiol 37(4):251–258. https://doi.org/10.1002/jobm.3620370404
Noreiga L, Guiemonde M, Sanchez B, Margolles A, de los Reyes Gavilan CG (2004) Effect of the adaptation to high bile salts concentrations on glycosidic activity, survival at low pH and cross-resistance to bile salts in Bifidobacterium. Int J Food Microbiol 94(1):79–86. https://doi.org/10.1016/j.ijfoodmicro.2004.01.003
Maus JE, Ingham SC (2003) Employment of stressful conditions during culture production to enhance subsequent cold- and acid-tolerance of bifidobacteria. J Appl Microbiol 95(1):146–154. https://doi.org/10.1046/j.1365-2672.2003.01954.x
Saarela M (2004) Stationary-phase acid and heat pretreatments for improvement of the viability of probiotic lactobacilli and bifidobacteria. J Appl Microbiol 96(6):1205–1214. https://doi.org/10.1111/j.1365-2672.2004.02286.x
Kullen MJ, Klaenhammer TR (2000) Genetic modification of intestinal lactobacilli and bifidobacteria. Cur Issues Mol Biol 2(2):41–50
Sanchez B, Ruiz L, Gueimonde M, Ruas-Madiedo P, Margolles A (2012) Toward improving technological and functional properties of probiotics in foods. Trends Food Sci Technol 26(1):56–63. https://doi.org/10.1016/j.tifs.2012.02.002
WHO and Food and Agriculture organization of the United Nations Report (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. http://mesanders.com/probio_report.pdf
Weiss G, Jespersen L (2010) Transcriptional analysis of genes associated with stress and adhesion in Lactobacillus acidophilus NCFM during the passage through an in vitro gastrointestinal tract model. J Mol Microbiol Biotechnol 18(4):206–214. https://doi.org/10.1159/000316421
Calderini E, Celebioglu HU, Villarroel J, Jacobsen S, Svensson B, Pessione E (2017) Comparative proteomics of oxidative stress response of Lactobacillus acidophilus NCFM reveals effects on DNA repair and cysteine de novo synthesis. Proteomics 17(5). https://doi.org/10.1002/pmic.201600178
Lorca GL, Valdez GF (2001) A low-pH inducible, stationary-phase acid tolerance response in Lactobacillus acidophilus CRL-639. Curr Microbiol 42(1):21–25. https://doi.org/10.1007/s002840010172
Corcoran BM, Stanton C, Fitzgerald GF, Ross RP (2005) Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol 71(6):3060–3067. https://doi.org/10.1128/AEM.71.6.3060-3067.2005
Kim WS, Perl L, Park JH, Tandianus JE, Dunn NW (2001) Assessment of stress response of the probiotic Lactobacillus acidophilus. Curr Microbiol 43(5):346–350. https://doi.org/10.1007/s002840010314
Foster JW, Spector MP (1995) How Salmonella survive against the odds? Annu Rev Microbiol 49:145–174. https://doi.org/10.1007/s002840010314
Hecker M, Schumann W, Volker U (1996) Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol 19(3):417–428. https://doi.org/10.1046/j.1365-2958.1996.396932.x
Kurtmann L, Carlsen CU, Risbo J, Skibsted LH (2009) Storage stability of freeze-dried Lactobacillus acidophilus (la-5) in relation to water activity and presence of oxygen and ascorbate. Cryobiology 58(2):175–180. https://doi.org/10.1016/j.cryobiol.2008.12.001
FAO/WHO (2002) Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada, April 30 and May 1. http://www.who.int/foodsafety/publications/fs_management/probiotics2/en/
IDF (1992) General standards of Identity for Fermented Milks, Standard No. 163, International Dairy Federation, Brussels
Flahaut SA, Benachour A, Giard J, Boutibonnes P, Auffray Y (1996) Defence against lethal treatments and de novo protein synthesis induced by NaCl in Enterococcus faecalis ATCC 19433. Arch Microbiol 165(5):317–324. https://doi.org/10.1007/s002030050333
Broadbent JR, Oberg CJ, Wang H, Wei L (1997) Attributes of the heat shock response in three species of dairy Lactobacillus. Syst Appl Microbiol 20(1):12–19. https://doi.org/10.1016/S0723-2020(97)80043-4
Cotter PD, Hill C (2003) Surviving the acid test: reponses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67(3):429–453. https://doi.org/10.1128/MMBR.67.3.429-453.2003
Wang H, Li Y, Zhang Y, Xi Q, Cao Y, Lu F (2017) Heat acclimation of Bifidobacterium longum and proteomic changes behind it. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-017-9251-4
Acknowledgements
The financial support from Small Business Innovation Research Initiative, Department of Biotechnology, India (DBT sanction order no. BT/SBIRI-1225/SBIRI-24/14), is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human or animal subjects.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kulkarni, S., Haq, S.F., Samant, S. et al. Adaptation of Lactobacillus acidophilus to Thermal Stress Yields a Thermotolerant Variant Which Also Exhibits Improved Survival at pH 2. Probiotics & Antimicro. Prot. 10, 717–727 (2018). https://doi.org/10.1007/s12602-017-9321-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9321-7