Abstract
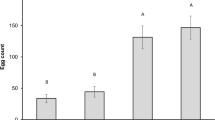
Insect-borne viruses promote several changes in plant phenotype, which can modify plant-vector interactions in favor of virus survival and dissemination. Although co-infections commonly occur in the field, little is known about their effects on interactions with the vector. The ecological interactions between Barley Yellow Dwarf Virus (BYDV) and its aphid vector, Rhopalosiphum padi, have been investigated extensively, but the vector’s behavior in more complex scenarios has yet to be examined. We assessed olfactory response and performance of R. padi to wheat singly and doubly infected by the pathogenic fungus Giberella zeae and BYDV. Non-viruliferous aphids preferred odors of BYDV-infected wheat over healthy wheat, as previously reported in the literature, and they were still preferentially attracted to BYDV-infected plant during co-infection. However, around 35% more non-viruliferous aphids chose healthy wheat over G. zeae-infected wheat. Viruliferous aphids did not show any preference to the treatments. BYDV-infected wheat was a superior host than healthy wheat for the aphids whose population increased in 25%. We observed a synergistic effect of the co-infected wheat, which was the best host for aphids, and promoted an elevation of 42% on population growth. Our results indicate that co-infection might be beneficial for virus spread as does not interfere with aphid olfactory preference and provides greater colony growth than in singly infected plants.



Similar content being viewed by others
References
Ajayi, O. (1986). The effect of barley yellow dwarf virus on the amino acid composition of spring wheat. Annals of Applied Biology, 108, 145–149.
Ajayi, B. O., & Dewar, A. M. (1983). The effect of barley yellow dwarf virus on field populations of the cereal aphids, Sitobion avenae and Metopolophium dirhodum. Annals of Applied Biology, 103, 1–11.
Alfenas, A. C., & Mafia, R. G. (2007). Métodos em fitopatologia. Viçosa: Editora UFV.
Araya, J. E., & Foster, J. E. (1987). Laboratory study on the effects of barley yellow dwarf virus on the life cycle of Rhopalosiphum padi (L.). Journal of Plant Diseases and Protection, 94, 578–583.
Bhat, R., Rai, R. V., & Karim, A. A. (2010). Mycotoxins in food and feed: present status and future concerns. Comprehensive Reviews in Food Science and Food Safety, 9, 57–81.
Bosque-Pérez, N. A., & Eigenbrode, S. D. (2011). The influence of virus-induced changes in plants on aphid vectors: insights from luteovirus pathosystems. Virus Research, 159, 201–205.
Brault, V., Uzest, M., Monsion, B., Jacquot, E., & Blanc, S. (2010). Aphids as transport devices for plant viruses. Comptes Rendus Biologies, 333, 524–538.
Casa, R. T., Reis, E. M., & Blum, M. M. C. (2004). Danos causados pela infecção de Gibberella zeae em trigo. Fitopatologia Brasileira, 29, 289–293.
Colvin, J. C. A., Omongo, M. R., Govindappa, P. C., Stevenson, M. N., Maruthi, G. G., Seal, S. E., & Muniyappa, V. (2006). Host-plant viral infection effects on arthropod-vector population growth, development and behavior: management and epidemiological implications. Advances in Virus Research, 67, 419–452.
Eckel, R. V. W., & Lampert, E. P. (1996). Relative attractiveness of tobacco etch virus-infected and healthy flue-cured tobacco plants to aphids (Homoptera: Aphididae). Journal of Economic Entomology, 89, 1017–1027.
Eigenbrode, S. D., Ding, H., Shiel, P., & Berger, P. H. (2002). Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proceedings of the Royal Society of London Series B-Biological Sciences, 269, 455–460.
Fereres, A., & Moreno, A. (2009). Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Research, 141, 158–168.
Gildow, F. E. (1980). Increased production of alatae by aphids reared on oats infected with barley yellow dwarf virus. Annals of Entomological Society of America, 73, 343–347.
Gray, S., & Gildow, F. E. (2003). Luteovirus-aphid interactions. Annual Review of Phytopathology, 41, 539–566.
Gray, S., Cilia, M., & Ghanim, M. (2014). Circulative, "Nonpropagative" virus transmission: an orchestra of virus-, insect-, and plant-derived instruments. Advances in Virus Research, 89, 141–199.
Hodge, S., & Powell, G. (2008). Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environmental Entomology, 37, 1573–1581.
Ingwell, L. L., Eigenbrode, S. D., & Bosque-Pérez, N. A. (2012). Plant viruses alter insect behavior to enhance their spread. Scientific Reports. doi:10.1038/srep00578.
Irwin, M. E., & Thresh, J. M. (1990). Epidemiology of barley yellow dwarf: a study in ecological complexity. Annual Review of Phytopathology, 28, 393–424.
Jiménez-Martinez, E. S., & Bosque-Pérez, N. A. (2004). Variation in barley yellow dwarf virus transmission efficiency by Rhopalosiphum padi (Homoptera: Aphididae) after acquisition from transgenic and nontransformed wheat genotypes. Journal of Economic Entomology, 97, 1790–1796.
Jiménez-Martínez, E. S., Bosque-Pérez, N. A., Berger, P. H., Zemetra, R. S., Ding, H., & Eigenbrode, S. D. (2004a). Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus-infected transgenic and untransformed wheat. Environmental Entomology, 33, 1207–1216.
Jiménez-Martínez, E. S., Bosque-Pérez, N. A., Berger, P. H., & Zemetra, R. S. (2004b). Life history of the bird cherry-oat aphid, Rhopalosiphum padi (Homoptera: Aphididae), on transgenic and untransformed wheat challenged with barley yellow dwarf virus. Journal of Economic Entomology, 97, 203–212.
Jing‐Quan, G. U. O., Lapierre, H., & Moreau, J. P. (1997). Vectoring ability of aphid clones of Rhopalosiphum padi (L.) and Sitobion avenae (Fabr.) and their capacity to retain barley yellow dwarf virus. Annals of Applied Biology, 131, 179–188.
Karyeija, R. F., Kreuze, J. F., Gibson, R. W., & Valkonen, J. P. T. (2000). Synergistic interactions of a potyvirus and a phloem-limited crinivirus in sweet potato plants. Virology, 269, 26–36.
Mahuku, G. S., Hall, R., & Goodwin, P. H. (1996). Co-infection and induction of systemic acquired resistance by weakly and highly virulent isolates of Leptosphaeria maculans in oilseed rape. Physiology and Molecular Biology of Plants, 49, 61–72.
Malpica, J. M., Sacristán, S., Fraile, A., & García-Arenal, F. (2006). Association and host selectivity in multi-host pathogens. PLoS ONE, 1, e41.
Mauck, K. E., De Moraes, C. M., & Mescher, M. C. (2010). Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proceedings of the National Academy of Sciences of the United States of America, 107, 3600–3605.
Mauck, K., Bosque‐Pérez, N. A., Eigenbrode, S. D., De Moraes, C. M., & Mescher, M. C. (2012). Transmission mechanisms shape pathogen effects on host–vector interactions: evidence from plant viruses. Functional Ecology, 26, 1162–1175.
McMullen, M., Jones, R., & Gallenberg, D. (1997). Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Disease, 81, 1340–1348.
Medina-Ortega, K. J., Bosque-Pérez, N. A., Ngumbi, E., Jiménez-Martínez, E. S., & Eigenbrode, S. D. (2009). Rhopalosiphum padi (Hemiptera: Aphididae) responses to volatile cues from barley yellow dwarf virus-infected wheat. Environmental Entomology, 38, 836–845.
Miller, W. A., & Rasochova, L. (1997). Barley yellow dwarf viruses. Annual Review of Phytopathology, 35, 167–190.
Moreno-Delafuente, A., Garzo, E., Moreno, A., & Fereres, A. (2013). A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. Plos One. doi:10.1371/journal.pone.0061543.
Müller, C. B., Williams, I. S., & Hardie, J. (2001). The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecological Entomology, 26, 330–340.
Parizoto, G., Rebonatto, A., Schons, J., & Lau, D. (2013). Barley yellow dwarf virus-PAV in Brazil: seasonal fluctuation and biological characteristics. Tropical Plant Pathology, 38, 11–19.
Pinto, Z. V., Rezende, J. A. M., Yuki, V. A., & Piedade, S. M. S. (2008). Ability of Aphis gossypii and Myzus persicae to Transmit cucumber mosaic virus in single and mixed infection with two potyviruses to zucchini squash. Summa Phytopathologica, 34, 183–185.
Potter, L. R. (1980). The effects of barley yellow dwarf virus and powdery mildew in oats and barley with single and dual infections. Annals of Applied Biology, 94, 11–17.
Salvaudon, L., De Moraes, C. M., & Mescher, M. C. (2013). Outcomes of co-infection by two potyviruses: implications for the evolution of manipulative strategies. Proceedings of the Royal Society of London Series B-Biological Sciences, 280, 2012–2959.
Simpson, D. R., Weston, G. E., Turner, J. A., Jennings, P., & Nicholson, P. (2001). Differential control of head blight pathogens of wheat by fungicides and consequences for mycotoxin contamination of grain. European Journal of Plant Pathology, 107, 421–431.
Smith, H. C. (1962). Is barley yellow dwarf virus a predisposing factor in the common root rot disease of wheat in Canada. Canadian Plant Disease Survey, 42, 143–148.
Srinivasan, R., & Alvarez, J. M. (2007). Effect of mixed viral infections (Potato virus Y - Potato leafroll virus) on biology and preference of vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). Journal of Economic Entomology, 100, 646–655.
Sward, R. M., & Kollmorgen, J. F. (1986). The separate and combined effects of barley yellow dwarf virus and take-all fungus (Gaeumannomyces graminis var. tritici) on the growth and yield of wheat. Crop Pasture Science, 37, 11–22.
Syller, J. (2012). Facilitative and antagonistic interactions between plant viruses in mixed infections. Molecular Plant Pathology, 13, 204–216.
Wintermantel, W. M. (2005). Co-infection of beet mosaic virus with beet yellowing viruses leads to increased symptom expression on sugar beet. Plant Disease, 89, 325–331.
Zadoks, J. C., Chang, T. T., & Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Research, 14, 415–421.
Acknowledgements
We thank Alfred Stoetzer (Fundação Agrária de Pesquisa Agropecuária, Guarapuava, PR) for providing seeds and the G. zeae isolate. We also thank Dr. Paulo Roberto Valle da Silva Pereira and Dr. Douglas Lau (Embrapa Trigo, Passo Fundo, RS) for providing the BYDV-PAV isolate and aphids. Felipe Goulart gently provided the schematic diagram of the dual choice set-up. This work was supported by the National Institute of Science and Technology (INCT) Semiochemicals in Agriculture, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP). MFGVP and PAS are supported by FAPESP (Process 2012/12252-1 and 2013/11993-0).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Infection by Barley yellow dwarf virus (BYDV) in wheat was verified by typical yellowing and reddening of leaves after two weeks of inoculation using viruliferous aphids (a); Giberella zeae infection was confirmed by cutting the stems of seed-inoculated wheat and leaving on a humid chamber for 48h. Thereafter, white mycelia (b) were collected and G. zeae was isolated in PDA medium. (DOCX 179 kb)
Online Resource 2
Schematic diagram of the aphid dual-choice arena. It consisted of a Petri dish (15 cm diameter) with two rectangular holes in the bottom covered with voile fabric to prevent the aphids from inserting their stylets into the plant tissue. Without being excised, a pair of leaves from each tested a plant were positioned below the Petri dish bottom and between the holes and a cardboard support. Twenty aphids in a circular filter paper were released in the center of the arena. (a) lateral view of the set-up; (b) top view of the arena showing aphids released in the center and the rectangular holes covered with voile fabric. (DOCX 164 kb)
Online Resource 3
Statistical data of dual choice bioassays with non-viruliferous (NV) and viruliferous (V) Rhopalosiphum padi wingless aphids analyzed by general log-linear model (treatment, time and treatment x time effect). (DOCX 19 kb)
Rights and permissions
About this article
Cite this article
dos Santos, R.C., Peñaflor, M.F.G.V., Sanches, P.A. et al. The effects of Gibberella zeae, Barley Yellow Dwarf Virus, and co-infection on Rhopalosiphum padi olfactory preference and performance. Phytoparasitica 44, 47–54 (2016). https://doi.org/10.1007/s12600-015-0493-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-015-0493-y