Abstract
Purpose of Review
The present review focus on the published literature about the use of 18F-fluorodeoxyglucose (FDG) PET/CT imaging in the early recognition of anthracyclines-related cardiotoxicity.
Recent Findings
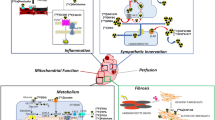
The application of PET/CT may represent an early predictor of subsequent cardiotoxicity in cancer patients treated with doxorubicin (DXR). However, the application of PET/CT may also extend beyond mere cardiotoxicity identification and monitoring to provide mechanistic delineation of the cardiotoxic pathophysiology. Indeed, this tool further enriched the current knowledge on energy metabolism impairment in the DXR-induced cardiotoxic cascade.
Summary
The capability of FDG to selectively track the early endoplasmic reticulum pentose phosphate pathway (PPP) response to oxidative stress rather than the later occurring contractile dysfunction might imply the abrupt occurrence of metabolic abnormality during the course of chemotherapy, possibly identifying the ongoing myocardial damage in time to change the chemotherapy scheme or to initiate targeted cardioprotective treatments. Future prospective studies encompassing a specific dietary or pharmacologic preparation before FDG injection, as already performed in infectious and inflammatory heart diseases, are needed to move the obtained preclinical findings supporting the role of FDG imaging in DXR cardiotoxicity from bench to bedside.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ellison LF. Progress in net cancer survival in Canada over 20 years. Health Rep. 2018;29:10–8.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2016;66:271–89. https://doi.org/10.3322/caac.21349.
Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell. 2008;7:125–36.
• Mercurio V, Pirozzi F, Lazzarini E, Marone G, Rizzo P, Agnetti G, et al. Models of heart failure based on the cardiotoxicity of anticancer drugs. J Card Fail. 2016;22:449–58. https://doi.org/10.1016/j.cardfail.2016.04.008. Interesting review of the proposed models of heart failure based on the cardiotoxicity of antineoplastic drugs, including the heart failure resulting from the combination of oxidative stress, mitochondrial dysfunction, and activation of the DNA damage response, which is typically caused by anthracyclines.
Dickey JS, Rao VA. Current and proposed biomarkers of anthracycline cardiotoxicity in cancer: emerging opportunities in oxidative damage and autophagy. Curr Mol Med. 2012;12:763–71.
Marshall RC, Tillisch JH, Phelps ME, Huang SC, Carson R, Henze E, et al. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation. 1983;67:766–78. https://doi.org/10.1161/01.cir.67.4.766.
Inglese E, Leva L, Matheoud R, Sacchetti G, Secco C, Gandolfo P, et al. Spatial and temporal heterogeneity of regional myocardial uptake in patients without heart disease under fasting conditions on repeated whole-body 18F-FDG PET/CT. J Nucl Med. 2007;48:1662–9. https://doi.org/10.2967/jnumed.107.041574.
Borde C, Kand P, Basu S. Enhanced myocardial fluorodeoxyglucose uptake following Adriamycin-based therapy: evidence of early chemotherapeutic cardiotoxicity? World J Radiol. 2012;4:220–3. https://doi.org/10.4329/wjr.v4.i5.220.
•• Bauckneht M, Ferrarazzo G, Fiz F, Morbelli S, Sarocchi M, Pastorino F, et al. Doxorubicin effect on myocardial metabolism as a prerequisite for subsequent development of cardiac toxicity: a translational 18F-FDG PET/CT observation. J Nucl Med. 2017;58:1638–45. https://doi.org/10.2967/jnumed.117.191122. The first clinical observation of the correlation between myocardial FDG uptake and cardiotoxicity in a group of HL patients successfully treated with DXR.
Sarocchi M, Bauckneht M, Arboscello E, Capitanio S, Marini C, Morbelli S, et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J Transl Med. 2018;16:295. https://doi.org/10.1186/s12967-018-1670-9.
•• Kim J, Cho SG, Kang SR, Yoo SW, Kwon SY, Min JJ, et al. Association between FDG uptake in the right ventricular myocardium and cancer therapy-induced cardiotoxicity. J Nucl Cardiol. 2019. https://doi.org/10.1007/s12350-019-01617-y. The correlation between myocardial FDG uptake and the subsequent development of cardiotoxicity in a group of breast cancer patients treated with anthracyclines or trastuzumab, further suggesting that oncologic FDG PET/CT scans can provide information regarding cancer therapy-induced cardiotoxicity as well as tumor response.
Keramida K, Farmakis D, Bingcang J, Sulemane S, Sutherland S, Bingcang RA, et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail. 2019;21:529–35. https://doi.org/10.1002/ejhf.1385.
Hrelia S, Fiorentini D, Maraldi T, Angeloni C, Bordoni A, Biagi PL, et al. Doxorubicin induces early lipid peroxidation associated with changes in glucose transport in cultured cardiomyocytes. Biochim Biophys Acta. 2002;1567:150–6. https://doi.org/10.1016/s0005-2736(02)00612-0.
Carvalho RA, Sousa RP, Cadete VJ, Lopaschuk GD, Palmeira CM, Bjork JA, et al. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology. 2010;270:92–8. https://doi.org/10.1016/j.tox.2010.01.019.
Tokarska-Schlattner M, Zaugg M, da Silva R, Lucchinetti E, Schaub MC, Wallimann T, et al. Acute toxicity of doxorubicin on isolated perfused heart: response of kinases regulating energy supply. Am J Physiol Heart Circ Physiol. 2005;289:H37–47. https://doi.org/10.1152/ajpheart.01057.2004.
•• Bauckneht M, Pastorino F, Castellani P, Cossu V, Orengo AM, Piccioli P, et al. Increased myocardial 18F-FDG uptake as a marker of doxorubicin-induced oxidative stress. J Nucl Cardiol. 2019. https://doi.org/10.1007/s12350-019-01618-x The preclinical data supporting the pathophysiological interpretation of the increased myocardial FDG uptake following DXR exposure.
Clarke JL, Mason PJ. Murine hexose-6-phosphate dehydrogenase: a bifunctional enzyme with broad substrate specificity and 6-phosphogluconolactonase activity. Arch Biochem Biophys. 2003;415:229–34. https://doi.org/10.1016/s0003-9861(03)00229-7.
Cossu V, Marini C, Piccioli P, Rocchi A, Bruno S, Orengo AM, et al. Obligatory role of endoplasmic reticulum in brain FDG uptake. Eur J Nucl Med Mol Imaging. 2019;46:1184–96. https://doi.org/10.1007/s00259-018-4254-2.
Buschiazzo A, Cossu V, Bauckneht M, Orengo A, Piccioli P, Emionite L, et al. Effect of starvation on brain glucose metabolism and 18F-2-fluoro-2-deoxyglucose uptake: an experimental in-vivo and ex-vivo study. EJNMMI Res. 2018;8:44. https://doi.org/10.1186/s13550-018-0398-0.
Marini C, Ravera S, Buschiazzo A, Bianchi G, Orengo AM, Bruno S, et al. Discovery of a novel glucose metabolism in cancer: the role of endoplasmic reticulum beyond glycolysis and pentose phosphate shunt. Sci Rep. 2016;6:25092. https://doi.org/10.1038/srep25092.
Marini C, Bianchi G, Buschiazzo A, Ravera S, Martella R, Bottoni G, et al. Divergent targets of glycolysis and oxidative phosphorylation result in additive effects of metformin and starvation in colon and breast cancer. Sci Rep. 2016;6:19569. https://doi.org/10.1038/srep19569.
Scussolini M, Bauckneht M, Cossu V, Bruno S, Orengo AM, Piccioli P, et al. G6Pase location in the endoplasmic reticulum: implications on compartmental analysis of FDG uptake in cancer cells. Sci Rep. 2019;9:2794. https://doi.org/10.1038/s41598-019-38973-1.
•• Tsachaki M, Mladenovic N, Štambergová H, Birk J, Odermatt A. Hexose-6-phosphate dehydrogenase controls cancer cell proliferation and migration through pleiotropic effects on the unfolded-protein response, calcium homeostasis, and redox balance. FASEB J. 2018;32:2690–705. https://doi.org/10.1096/fj.201700870RR This study shows the mechanism through which H6PD exerts its effects by H6PD silencing in cancer cells. Knockdown of H6PD resulted in an increase in ER lumen oxidation, and an increase in sarco/endoplasmic reticulum Ca2+-ATPase-2 pump expression and decrease in inositol trisphosphate receptor-III, which led to augmented levels of calcium in the ER, suggesting the strict connection between H6PD enzymatic activity, reticular antioxidant response and calcium homeostasis.
Salvemini F, Franze A, Iervolino A, Filosa S, Salzano S, Ursini MV. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J Biol Chem. 1999;274:2750–7. https://doi.org/10.1074/jbc.274.5.2750.
Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P, et al. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J. 2003;370:935–43. https://doi.org/10.1042/BJ20021614.
Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, et al. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004;11:823–31. https://doi.org/10.1038/sj.cdd.4401420.
Rogoff D, Black K, McMillan DR, White PC. Contribution of hexose-6-phosphate dehydrogenase to NADPH content and redox environment in the endoplasmic reticulum. Redox Rep. 2010;15:64–70. https://doi.org/10.1179/174329210X12650506623249.
Paik JY, Jung KH, Lee JH, Park JW, Lee KH. Reactive oxygen species-driven HIF1α triggers accelerated glycolysis in endothelial cells exposed to low oxygen tension. Nucl Med Biol. 2017;45:8–14. https://doi.org/10.1016/j.nucmedbio.2016.10.006.
Yan R, Song J, Wu Z, Guo M, Liu J, Li J, et al. Detection of myocardial metabolic abnormalities by 18F-FDG PET/CT and corresponding pathological changes in beagles with local heart irradiation. Korean J Radiol. 2015;16:919–28. https://doi.org/10.3348/kjr.2015.16.4.919.
Evans JD, Gomez DR, Chang JY, Gladish GW, Erasmus JJ, Rebueno N, et al. Cardiac 18F-fluorodeoxyglucose uptake on positron emission tomography after thoracic stereotactic body radiation therapy. Radiother Oncol. 2013;109:82–8. https://doi.org/10.1016/j.radonc.2013.07.021.
Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, et al. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H660–6. https://doi.org/10.1152/ajpheart.00268.2005.
Bulten BF, Sollini M, Boni R, Massri K, de Geus-Oei LF, van Laarhoven HWM, et al. Cardiac molecular pathways influenced by doxorubicin treatment in mice. Sci Rep. 2019;9:2514. https://doi.org/10.1038/s41598-019-38986-w.
Seino H, Ono S, Miura H, Morohashi S, Wu Y, Tsushima F, et al. Hypoxia is important in F-18 FDG accumulation in thecoma-fibroma tumors on F-18 FDG PET/CT scans. Mol Med Rep. 2016;13:3821–7. https://doi.org/10.3892/mmr.2016.5016.
Todorova VK, Beggs ML, Delongchamp RR, Dhakal I, Makhoul I, Wei JY, et al. Transcriptome profiling of peripheral blood cells identifies potential biomarkers for doxorubicin cardiotoxicity in a rat model. PLoS One. 2012;7:e48398. https://doi.org/10.1371/journal.pone.0048398.
Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I, et al. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial 18F fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol. 2016;23:244–52. https://doi.org/10.1007/s12350-015-0226-0.
Chen W, Sajadi MM, Dilsizian V. Merits of FDG PET/CT and functional molecular imaging over anatomic imaging with echocardiography and CT angiography for the diagnosis of cardiac device infections. JACC Cardiovasc Imaging. 2018;11:1679–91. https://doi.org/10.1016/j.jcmg.2018.08.026.
Genovesi D, Bauckneht M, Altini C, Popescu CE, Ferro P, Monaco L, et al. The role of positron emission tomography in the assessment of cardiac sarcoidosis. Br J Radiol. 2019;92:20190247. https://doi.org/10.1259/bjr.20190247.
Bauckneht M, Morbelli S, Fiz F, Ferrarazzo G, Piva R, Nieri A, Sarocchi M, Spallarossa P, Canepari M, Arboscello E, Bellodi A, Massaia M, Gallamini A, Bruzzi P, Marini C, Sambuceti G A score-based approach to 18F-FDG PET images as a tool to describe metabolic predictors of myocardial doxorubicin susceptibility. Diagnostics (Basel) 2017;7. Doi: https://doi.org/10.3390/diagnostics7040057.
Keyes JW. SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiac Nuclear Imaging
Rights and permissions
About this article
Cite this article
Bauckneht, M., Cossu, V., Miceli, A. et al. FDG-PET Imaging of Doxorubicin-Induced Cardiotoxicity: a New Window on an Old Problem. Curr Cardiovasc Imaging Rep 12, 41 (2019). https://doi.org/10.1007/s12410-019-9517-1
Published:
DOI: https://doi.org/10.1007/s12410-019-9517-1