Abstract
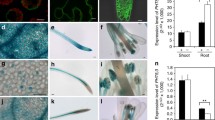
An efficient monitoring of cytoplasmic vacuolation by fluorescein diacetate (FDA) staining of protoplasts revealed that the major populations of suspension-cultured rice cells undergo a rapid vacuolation upon glucosedepletion. As aquaporin-family proteins, tonoplast intrinsic proteins (TIPs) are known to play in regulating the water balance across the vacuolar membrane. Glucose starvation increased expression of every member of OsTIP family, leading to an enhancement of the total expression by up to 110-fold, which is well matched with an expansion of the vacuolar structure induced by starvation. OsTIPs 1;1, 2;2 and 3;1 are the three most prominently expressed OsTIPs in starved conditions due to their highest responsiveness to sugar deprivation. Feeding experiments with various sugars and glucose analogs indicated that sugar regulated expression of the three major OsTIPs is likely mediated by a hexokinasedependent pathway. Alleviation of sugar-induced suppression of OsTIP expression by co-treatment with the uncoupler of ATP synthesis suggests that sugar signaling for OsTIP regulation is also cross-talked by the energy-deficit conditions. Intriguingly, starvation-induced central vacuolation was effectively prevented by mannose and 2-deoxyglucose, but not by 3-O-methylglucose. These results imply that the hexokinase is able to trigger the signaling to suppress the central vacuolation, independently of fueling the energy metabolism.
Similar content being viewed by others
References
Amelunxen F, Heinze U (1984) Zur entwicklung der vacuole in testazellen des Leinsamens (On the development of the vacuole in the testa cells on Linum seeds). Eur J Cell Biol 35:343–354
Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol 133:1251–1263.
Baker A, Graham I (2002) Plant peroxisomes: Biochemistry, cell biology and biotechnological applications. Ed 1, Springer Netherlands, Dordrecht, p505
Borek S, Morkunas I, Ratajczak W, Ratajczak L (2001) Metabolism of amino acids in germinating yellow lupin seeds. III Breakdown of arginine in sugar-starved organs cultivated in vitro. Acta Physiol Plant 23:141–148
Brouquisse R, Evrard A, Rolin D, Raymond P, Roby C (2001) Regulation of protein degradation and protease expression by mannose in maize root tips. Pi sequestration by mannose may hinder the study of its signaling properties. Plant Physiol 125: 1485–1498
Brouquisse R, Gaudillère J, Raymond P (1998) Induction of a carbonstarvation-related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiol 117: 1281–1291
Brouquisse R, James F, Pradet A, Raymond P (1992) Asparagine metabolism and nitrogen distribution during protein degradation in sugar-starved maize root tips. Planta 188:384–395
Brouquisse R, James F, Rajmond P, Pradet A (1991) Study of glucose starvation in excised maize root tips. Plant Physiol 96:619–626
Chen MH, Liu LF, Chen YR, Wu HK, Yu SM (1994) Expression of a-amylases, carbohydrate metabolism, and autophagy in cultured rice cells are coordinately regulated by sugar nutrient. Plant J 6:625–636
Chrispeels MJ, Maurel C (1994) Aquaporins: the molecular basis of hydraulic water movement between cells? Plant Physiol 105:9–13
Elamrani A, Gaudillère J, Raymond P (1994) Carbohydrate starvation is a major determinant of the loss of greening capacity in cotyledons of dark-grown sugar beet seedlings. Physiol Plant 91: 56–64
Escobar-Gutiérrez AJ, Zipperlin B, Carbonne F, Moing A, Gaudillére JP (1998) Photosynthesis, carbon partitioning and metabolite content during drought stress in peach seedlings. Australian J Plant Physiol 25:197–205
Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P (2006) Identification of sugar modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res 119:115–123
Granot D (2008) Putting plant hexokinases in their proper place. Phytochemistry 69:2649–2654
Higuchi T, Suga S, Tsuchiya T, Hisada H, Morishima S, Okada Y, Maeshima M (1998) Molecular cloning, water channel activity and tissue specific expression of two isoforms of radish vacuolar aquaporin. Plant Cell Physiol 39:905–913
Hilling B, Amelunxen F (1985) On the development of the vacuole. II. Further evidence for endoplasmic reticulum origin. Eur J Cell Biol 38:195–200
Hwang YS, Karrer EE, Thomas BR, Chen L, Rodriguez RL (1998) Three cis-elements required for rice α-amylase Amy3D expression during sugar starvation. Plant Mol Biol 36:331–341
Jang JC, Leon P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9:5–19
Jauh GY, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11:1867–1882
Jiang L, Phillips TE, Hamm CA, Drozdowicz YM, Rea PA, Maeshima M, Rogers SW, Rogers JC (2001) The protein storage vacuole: a unique compound organelle. J Cell Biol 155:991–1002
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126:1358–1369
Johnson KD, Herman EM, Chrispeels MJ (1989) An abundant, highly conserved tonoplast protein in seeds. Plant Physiol 91: 1006–1013
Journet EP, Bligny R, Douce R (1986) Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem 261: 3193–3199
Karlsson M, Johansson I, Bush M, McCann M, Maurel C, Larsson C, Kjellbom P (2000) An abundant TIP expressed in mature highly vacuolated cells. Plant J 21:83–90
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Kraemer GP, Alberte RS (1995) Impact of daily photosynthetic period on protein synthesis and carbohydrate stores in Zostera marina L. (eelgrass) roots: implications for survival in lightlimited environments. J Exp Mar Biol Ecol 185:191–202
Lee KW, Chen PW, Lu CA, Chen S, Ho TH, Yu SM (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci Signal 2, ra61
Li GW, Peng YH, Yu X, Zhang MH, Cai WM, Sun WN, Su WA (2008) Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. J Plant Physiol 165:1879–1888
Ligaba A, Katsuhara M, Shibasaka M, Djira G (2011) Abiotic stresses modulate expression of major intrinsic proteins in barley (Hordeum vulgare). Crit Rev Biol 334:127–139
Lim M, Lee S, Yim H, Kim J, Yoon I, Hwang YS (2013) Differential anoxic expression of sugar-regulated genes reveals diverse interactions between sugar and anaerobic signaling systems in rice. Mol Cells 36:169–176
Loque´ D, Ludewig U, Yuan L, Wirèn N (2005) Tonoplast Intrinsic Proteins AtTIP2;1 and AtTIP2;3 Facilitate NH3 Transport into the Vacuole. Plant Physiol 137:671–680
Loughman BC, Ratcliffe RG, Southon TE (1989) Observations on the cytoplasmic and vacuolar orthophosphate pools in leaf tissues using in vivo 31P-NMR spectroscopy. FEBS Lett 242:279–284
Ludevid D, Höfte H, Himelblau E, Chrispeels M (1992) The Expression Pattern of the Tonoplast Intrinsic Protein gamma-TIP in Arabidopsis thaliana Is Correlated with Cell Enlargement. Plant Physiol 100:1633–1639
Maegawa K, Takii R, Ushimaru T, Kozaki A (2015) Evolutionary conservation of TORC1 components, TOR, Raptor, and LST8, between rice and yeast. Mol Genet Genomics 290:2019–2030
Martinoia E, Maeshima M, Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58:83–102
Marty F (1978) Cytochemical studies on GERL, provacuoles, and vacuoles in root meristematic cells of Euphorbia. Proc Natl Acad Sci USA 75: 852–856
Marty F (1997) The biogenesis of vacuoles: Insights from microscopy. Adv Bot Res 25:1–42
Marty F (1999) Plant vacuoles. Plant Cell Physiol 11:587–599
Maurel C (1997) Aquaporins and water permeability of plant membranes. Annu Rev Plant Phys l 48:399–429
Maurel C, Chrispeels MJ, Lurin C, Tacnet F, Geelen D, Ripoche P, Guern J (1997) Function and regulation of seed aquaporins. J Exp Bot 48:421–430
Moriyasu Y, Hattori M, Jauh GY, Rogers JC (2003) Alpha tonoplast intrinsic protein is specifically associated with vacuole membrane involved in an autophagic process. Plant Cell Physiol 44:795–802
Moriyasu Y, Klionsky DJ (2004) Autophagy in Plants, In Klionsky DJ ed, Autophagy, Landes Bioscience Austin
Müntz K (2007) Protein dynamics and proteolysis in plant vacuoles. J Exp Bot 58:2391–2407
Okubo-Kurihara E, Sano T, Higaki T, Kutsuna N, Hasezawa S (2009) Acceleration of vacuolar regeneration and cell growth by overexpression of an aquaporin NtTIP1;1 in tobacco BY-2 cells. Plant Cell Physiol 50:151–160
Olbrich A, Hillmer S, Hinz G, Oliviusson P, Robinson DG (2007) Newly formed vacuoles in root meristems of barley and pea seedlings have characteristics of both protein storage and lytic vacuoles. Plant Physiol 145:1383–1394
Park M, Yim HK, Park HG, Lim J, Kim SH, Hwang YS (2010) Interference with oxidative phosphorylation enhances anoxic expression of rice alpha-amylase genes through abolishing sugar regulation. J Exp Bot 61:3235–3244
Ratajczak W, Lehmann T, Polcyn W, Ratajczak L (1996) Metabolism of amino acids in germinating yellow lupin seeds. I. The decomposition of 14C-aspartate and 114C-glutamate during the imbibition. Acta Physiol Plant 18:13–18
Roberts DJ, Tan-Sah VPY Ding EY, Jim S, Miyamoto S (2014) Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell 53:521–533
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Phys 57:675–709
Salas J, Salas M, Vinuela E, Sols A (1965) Gucokinase of rabbit liver. J Biol Chem 240:1014–1018
Schmelzle T, Hall MN (2000) TOR, a central controller of cell growth. Cell 103:253–262
Sheen J (2010) Discover and connect cellular signaling. Plant Physiol 154:562–566
Sheu-Hwa C-S, Lewis DH, Waker DA (1975) Stimulation of photosynthetic starch formation by sequestration of cytoplasmic orthophosphate. New Phytol 74:383–392
Thompson J, Abdullah R, Cocking E (1986) Protoplast culture of rice (Oryza sativa L.) using media solidified with agarose. Plant Sci 47:123–133
Vierstra RD (1996) Proteolysis in plants: mechanisms and functions. Plant Mol Biol 32:275–302
Xiao W, Sheen J, Jang JC (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44:451–461
Yu S (1999) Cellular and genetic responses of plants to sugar starvation. Plant Physiol 121:687–693
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, Mn., Choi, S., Lee, Se. et al. Sugar starvation induces the central vacuolation with coordinated increase in expression of tonoplast intrinsic protein genes in suspension-cultured rice cells. J. Plant Biol. 59, 74–82 (2016). https://doi.org/10.1007/s12374-016-0455-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-016-0455-z