Abstract
Introduction
Exogenous progesterone is prescribed for a variety of conditions with endogenous progesterone deficiency, e.g. menstrual alterations, primary or secondary infertility or premenopause. To the best of our knowledge, no pharmacogenetic studies have been published in relation to exogenous progesterone pharmacokinetic safety or progesterone metabolites so far.
Methods
Candidate-gene study where we evaluated whether five single-nucleotide polymorphisms (CYP2C9*2, *3, CYP2C19*2, *3 and *17) were related to the pharmacokinetics, safety and metabolism of progesterone in 24 healthy volunteers who received a 200-mg progesterone formulation either orally or vaginally.
Results
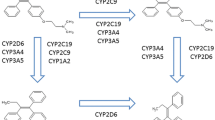
The vaginal formulation had an average AUCt value approximately 18 times greater than the oral formulation. CYP2C19 intermediate metabolizers (IM) consistently showed higher adjusted AUCt and adjusted Cmax than extensive metabolizers (EM) (P < 0.05); CYP2C9 EM incongruently exhibited higher adjusted Cmax and longer half-life than IM (p < 0.05).
Conclusion
This is the first study that reports variability in progesterone disposition according to the CYP2C19 and CYP2C9 phenotype. We suggest that CYP2C19 may condition progesterone disposition and that it may be more relevant than CYP2C9. This study lays the foundations for further in-depth research to evaluate the pharmacogenetics of progesterone.
Trial Registration
EudraCT numbers are 2012-005105-43 and 2012-005011-10.

Similar content being viewed by others
References
Fitzpatrick LA, Good A. Micronized progesterone: clinical indications and comparison with current treatments. Fertil Steril. 1999;72(3):389–97.
Dante G, Vaccaro V, Facchinetti F. Use of progestagens during early pregnancy. Facts Views Vis ObGyn. 2013;5(1):66–71.
Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1(1):50–62. https://doi.org/10.1016/j.ajogmf.2019.03.001.
Ficha técnica utrogestan 100 mg cápsulas blandas. Agencia Española del Medicamento y Productos Sanitarios. 2015. https://cima.aemps.es/cima/pdfs/es/ft/64899/FT_64899.html.pdf. Accessed 15 July 2019.
Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(sup1):3–63. https://doi.org/10.1080/13697130500148875.
Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68(10–13):879–90.
Yamazaki H, Shimada T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997;346(1):161–9. https://doi.org/10.1006/abbi.1997.0302.
Saiz-Rodríguez M, Romero-Palacián D, Villalobos-Vilda C, et al. Influence of CYP2C19 phenotype on the effect of clopidogrel in patients undergoing a percutaneous neurointervention procedure. Clin Pharmacol Ther. 2019;105(3):661–71. https://doi.org/10.1002/cpt.1067.
Cooper GM, Johnson JA, Langaee TY, et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112(4):1022–7. https://doi.org/10.1182/blood-2008-01-134247.
Klein K, Zanger UM. Pharmacogenomics of cytochrome P450 3A4: recent progress toward the “Missing Heritability” problem. Front Genet. 2013. https://doi.org/10.3389/fgene.2013.00012.
Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther. 1977;21(3):247–54.
Scordo MG, Aklillu E, Yasar U, Dahl M-L, Spina E, Ingelman-Sundberg M. Genetic polymorphism of cytochrome P450 2C9 in a Caucasian and a black African population: short report. Br J Clin Pharmacol. 2001;52(4):447–50. https://doi.org/10.1046/j.0306-5251.2001.01460.x.
Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the heart and estrogen/progestin replacement study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9.
Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33.
The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275(5):370–5.
Regidor P-A. Progesterone in peri- and postmenopause: a review. Geburtshilfe Frauenheilkd. 2014;74(11):995–1002. https://doi.org/10.1055/s-0034-1383297.
Paulson RJ, Collins MG, Yankov VI. Progesterone pharmacokinetics and pharmacodynamics with three dosages and two regimens of an effervescent micronized progesterone vaginal insert. J Clin Endocrinol Metab. 2014;99(11):4241–9. https://doi.org/10.1210/jc.2013-3937.
Preissner SC, Hoffmann MF, Preissner R, Dunkel M, Gewiess A, Preissner S. Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. Nie D, ed. PLoS One. 2013;8(12):e82562. https://doi.org/10.1371/journal.pone.0082562.
Zhou Y, Ingelman-Sundberg M, Lauschke VM. Worldwide distribution of cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Clin Pharmacol Ther. 2017;102(4):688–700. https://doi.org/10.1002/cpt.690.
Katz DF, Yuan A, Gao Y. Vaginal drug distribution modeling. Adv Drug Deliv Rev. 2015;92:2–13. https://doi.org/10.1016/j.addr.2015.04.017.
Jitendra N, Sharma PK, Bansal S, Banik A. Noninvasive routes of proteins and peptides drug delivery. Indian J Pharm Sci. 2011;73(4):367–75. https://doi.org/10.4103/0250-474x.95608.
Acknowledgements
The authors are thankful to the volunteers and the effort of the staff of the Clinical Trial Unit of Hospital Universitario de La Princesa.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Dolores Ochoa has been a consultant or investigator in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Cinfa, FAES Farma, Farmalíder, Ferrer, GlaxoSmithKline, Galenicum, Gilead, Italfarmaco, Janssen-Cilag, Kern Pharma, Normon, Novartis, Servier, Silverpharma, Teva and Zambon. Francisco Abad-Santos has been a consultant or investigator in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Cinfa, FAES Farma, Farmalíder, Ferrer, GlaxoSmithKline, Galenicum, Gilead, Italfarmaco, Janssen-Cilag, Kern Pharma, Normon, Novartis, Servier, Silverpharma, Teva and Zambon. Dora Koller is co-financed by the H2020 Marie Sklodowska-Curie Innovative Training Network 721236 grant. Pablo Zubiaur is co-financed by Consejería de Educación, Juventud y Deporte from Comunidad de Madrid and Fondo Social Europeo. Mª Ángeles Gálvez, Miriam Saiz-Rodriguez, Manuel Román, Mónica Aguilar and Itziar de Pablo have nothing to disclose.
Compliance with Ethics Guidelines
The clinical trial with the oral formulation was approved by the Clinical Research Ethics Committee of Hospital Universitario de La Princesa (Madrid). The clinical trial with the vaginal formulation was approved by the Clinical Research Ethics Committee of Hospital Universitario Ramón y Cajal (Madrid). Both studies were authorized by the Spanish Drugs Agency (AEMPS), were accomplished under the guidelines of Good Clinical Practice and complied with Spanish legislation on clinical research in humans. The study conformed with the Helsinki Declaration of 1964, as revised in 2013, concerning human and animal rights, and with Springer’s policy concerning informed consent. All subjects provided their informed consent for the clinical trial and pharmacogenetic study. EudraCT numbers are 2012-005105-43 and 2012-005011-10 for the vaginal and oral formulation respectively.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available as they belong to the sponsor of the clinical trial but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9692690.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zubiaur, P., Ochoa, D., Gálvez, M.Á. et al. Effect of Polymorphisms in CYP2C9 and CYP2C19 on the Disposition, Safety and Metabolism of Progesterone Administrated Orally or Vaginally. Adv Ther 36, 2744–2755 (2019). https://doi.org/10.1007/s12325-019-01075-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01075-5