Abstract
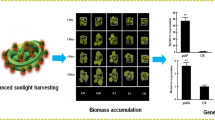
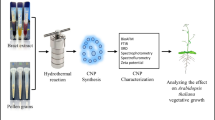
The ∼ 5 nm degradable carbon dots (CDs) were synthesized directly from carbon rod by a one-step electrochemical method at room temperature. The as-prepared CDs can effectively enhance the ribulose bisphosphate carboxylase oxygenase (RuBisCO) activity, and then promote the dicotyledons growth (soybean, tomato, eggplant and so on) and finally increase their yields. Here, we used Arabidopsis thaliana and Trifolium repens L. as model plants to systematically study the beneficial effects of CDs on plant growth. These include: (i) accelerating seed germination; (ii) enlarging root elongation; (iii) increasing metal ions absorption and delivery; (iv) improving enzymes activity; (v) enhancing the carbohydrate content; (vi) degradation into plant hormone analogues and CO2; and finally (vii) enhancing the grain production by about 20%.

Similar content being viewed by others
References
Hamilton, A.; Hamilton, P. Plant Conservation: An Ecosystem Approach; Earthscan: London, 2006.
Kesler, S. E.; Simon, A. C. Mineral Resources, Economics and the Environment; Cambridge University: Cambridge, 2015.
Zheng, L.; Hong, F. S.; Lu, S. P.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91.
Hong, F. S.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279.
Gao, F. Q.; Hong, F. S.; Liu, C.; Zheng, L.; Su, M. Y.; Wu, X.; Yang, F.; Wu, C.; Yang, P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach: Inducing complex of rubisco-rubisco activase. Biol. Trace Elem. Res. 2006, 111, 239–253.
Zheng, L.; Su, M. Y.; Liu, C.; Chen, L.; Huang, H.; Wu, X.; Liu, X. Q.; Yang, F.; Gao, F. Q.; Hong, F. S. Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol. Trace Elem. Res. 2007, 119, 68–76.
Gao, F. Q.; Liu, C.; Qu, C. X.; Zheng, L.; Yang, F.; Su, M. Y.; Hong, F. S. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of rubisco activase? BioMetals 2008, 21, 211–217.
Ma, L. L.; Liu, C.; Qu, C. X.; Yin, S. T.; Liu, J.; Gao, F. Q.; Hong, F. H. Rubisco activase mRNA expression in spinach: Modulation by nanoanatase treatment. Biol. Trace Elem. Res. 2008, 122, 168–178.
Wang, X. M.; Gao, F. Q.; Ma, L. L.; Liu, J.; Yin, S. T.; Yang, P.; Hong, F. S. Effects of nano-anatase on ribulose-1, 5-bisphosphate carboxylase/oxygenase mRNA expression in spinach. Biol. Trace Elem. Res. 2008, 126, 280–289.
Su, M. Y.; Liu, J.; Yin, S. T.; Ma, L. L.; Hong, F. S. Effects of nanoanatase on the photosynthetic improvement of chloroplast damaged by linolenic acid. Biol. Trace Elem. Res. 2008, 124, 173–183.
Yang, F.; Hong, F. S.; You, W. J.; Liu, C.; Gao, F. Q.; Wu, C.; Yang, P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190.
Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water, Air, Soil Pollut. 2009, 197, 143–148.
Husen, A.; Siddiqi, K. S. Carbon and fullerene nanomaterials in plant system. J. Nanobiotechnology 2014, 12, 16.
Qu, G. B.; Bai, Y. H.; Zhang, Y.; Jia, Q.; Zhang, W. D.; Yan, B. The effect of multiwalled carbon nanotube agglomeration on their accumulation in and damage to organs in mice. Carbon 2009, 47, 2060–2069.
Liu, Q. L.; Chen, B.; Wang, Q. L.; Shi, X. L.; Xiao, Z. Y.; Lin, J. X.; Fang, X. H. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010.
Kang, S.; Herzberg, M.; Rodrigues, D. F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413.
Tripathi, S.; Sonkar, S. K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181.
Sonkar, S. K.; Roy, M.; Babar, D. G.; Sarkar, S. Water soluble carbon nanoonions from wood wool as growth promoters for gram plants. Nanoscale 2012, 4, 7670–7675.
Tripathi, K. M.; Bhati, A., Singh, A.; Sonker, A. K.; Sarkar, S.; Sonkar, S. K. Sustainable changes in the contents of metallic micronutrients in first generation gram seeds imposed by carbon nano-onions: Life cycle seed to seed study. ACS Sustainable Chem. Eng. 2017, 5, 2906–2916.
Villagarcia, H.; Dervishi, E.; De Silva, K.; Biris, A. S.; Khodakovskaya, M. V. Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants. Small 2012, 8, 2328–2334.
Lahiani, M. H.; Dervishi, E.; Chen, J. H.; Nima, Z.; Gaume, A.; Biris, A. S.; Khodakovskaya, M. V. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973.
Lahiani, M. H.; Dervishi, E.; Ivanov, I.; Chen, J. H.; Khodakovskaya, M. Comparative study of plant responses to carbon-based nanomaterials with different morphologies. Nanotechnology 2016, 27, 265102.
Kole, C.; Kole, P.; Randunu, K. M.; Choudhary, P.; Podila, R.; Ke, P. C.; Rao, A. M.; Marcus, R. K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol. 2013, 13, 37.
Saxena, M.; Maity, S.; Sarkar, S. Carbon nanoparticles in “biochar” boost wheat (Triticum aestivum) plant growth. RSC Adv. 2014, 4, 39948–39954.
Li, H. T.; He, X. D.; Kang, Z. H.; Huang, H.; Liu, Y.; Liu, J. L.; Lian, S. Y.; Tsang, C. H. A.; Yang, X. B.; Lee, S. T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem., Int. Ed. 2010, 49, 4430–4434.
Li, H.; Guo, S. J.; Li, C. X.; Huang, H.; Liu, Y.; Kang, Z. H. Tuning laccase catalytic activity with phosphate functionalized carbon dots by visible light. ACS Appl. Mater. Interfaces 2015, 7, 10004–10012.
Abu-Ghosh, S.; Kumar, V. B.; Fixler, D.; Dubinsky, Z.; Gedanken, A.; Iluz, D. Nitrogen-doped carbon dots prepared from bovine serum albumin to enhance algal astaxanthin production. Algal Res. 2017, 23, 161–165.
Liu, X. J.; Liu, L. T.; Hu, X. J.; Zhou, S. Y.; Ankri, R.; Fixler, D.; Xie, Z. Multimodal bioimaging based on gold nanorod and carbon dot nano-hybrids as a novel tool for atherosclerosis detection. Nano Res. 2018, 11, 1262–1273.
Niu, Y. F.; Ling, G.; Wang, L.; Guan, S. Y.; Xie, Z.; Barnoy, E. A.; Zhou, S. Y.; Fixler, D. Gold rod-polyethylene glycol-carbon dot nanohybrids as phototheranostic probes. Nanomaterials 2018, 8, 706.
Guo, S. J.; Zhao, S. Q.; Wu, X. Q.; Li, H.; Zhou, Y. J.; Zhu, C.; Yang, N. J.; Jiang, X.; Gao, J.; Bai, L. et al. A Co3O4-CDots-C3N4 three component electrocatalyst design concept for efficient and tunable CO2 reduction to syngas. Nat. Commun. 2017, 8, 1828.
Liu, J.; Liu, Y.; Liu, N. Y.; Han, Y. Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S. T.; Zhong, J.; Kang, Z. H. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974.
Yu, H.; Du, L. B.; Guan, L. M.; Zhang, K.; Li, Y. Y.; Zhu, H. J.; Sun, M. T.; Wang, S. H. A ratiometric fluorescent probe based on the pi-stacked graphene oxide and cyanine dye for sensitive detection of bisulfite. Sens. Actuators B: Chem. 2017, 247, 823–829.
Yan, Y. H.; Yu, H.; Zhang, K.; Sun, M. T.; Zhang, Y. J.; Wang, X. K.; Wang, S. H. Dual-emissive nanohybrid of carbon dots and gold nanoclusters for sensitive determination of mercuric ions. Nano Res. 2016, 9, 2088–2096.
Ge, H. W.; Zhang, K.; Yu, H.; Yue, J.; Yu, L.; Chen, X. F.; Hou, T. X.; Alamry, K. A.; Marwani, H. M.; Wang, S. H. Sensitive and selective detection of antibiotic d-penicillamine based on a dual-mode probe of fluorescent carbon dots and gold nanoparticles. J. Fluoresc. 2018, 28, 1405–1412.
Li, H.; Kong, W. Q.; Liu, J.; Liu, N. Y.; Huang, H.; Liu, Y.; Kang, Z. H. Fluorescent N-doped carbon dots for both cellular imaging and highly-sensitive catechol detection. Carbon 2015, 91, 66–75.
Lu, F.; Song, Y. X.; Huang, H.; Liu, Y.; Fu, Y. J.; Huang, J.; Li, H.; Qu, H. H.; Kang, Z. H. Fluorescent carbon dots with tunable negative charges for bio-imaging in bacterial viability assessment. Carbon 2017, 120, 95–102.
Ge, J. C.; Lan, M. H.; Zhou, B. J.; Liu, W. M.; Guo, L.; Wang, H.; Jia, Q. Y.; Niu, G. L.; Huang, X.; Zhou, H. Y. et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596.
Lim, S. Y.; Shen, W.; Gao, Z. Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381.
Jermyn, M. A. Increasing the sensitivity of the anthrone method for carbohydrate. Anal. Biochem. 1975, 68, 332–335.
Loewus, F. A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 1952, 24, 219.
Hohl, H.; Stumm, W. Interaction of Pb2+ with hydrous γ-Al2O3. J. Colloid Interface Sci. 1976, 55, 281–288.
Crocker, M.; Herold, R. H. M.; Sonnemans, M. H. W.; Emeis, C. A.; Wilson, A. E.; Van Der Moolen, J. N. Studies on the acidity of mordenite and ZSM 5. 1. Determination of broensted acid site concentrations in mordenite and ZSM 5 by conductometric titration. J. Phys. Chem. 1993, 97, 432–439.
Hunt, J. P.; Taube, H. The photochemical decomposition of hydrogen peroxide. Quantum yields, tracer and fractionation effects. J. Am. Chem. Soc. 1952, 74, 5999–6002.
Zhao, Y.; Allen, B. L.; Star, A. Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A 2011, 115, 9536–9544.
Lan, Y.; Mott, K. A. Determination of apparent K m values for ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase using the spectrophotometric assay of rubisco activity. Plant Physiol. 1991, 95, 604–609.
Chong, Y.; Ma, Y. F.; Shen, H.; Tu, X. L.; Zhou, X.; Xu, J. Y.; Dai, J. W.; Fan, S. J.; Zhang, Z. J. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048.
Ming, H.; Ma, Z.; Liu, Y.; Pan, K. M.; Yu, H.; Wang, F.; Kang, Z. H. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531.
Li, H.; Zhang, M. L.; Song, Y. X.; Wang, H. B.; Liu, C. A.; Fu, Y. J.; Huang, H.; Liu, Y.; Kang, Z. H. Multifunctional carbon dot for lifetime thermal sensing, nucleolus imaging and antialgal activity. J. Mater. Chem. B 2018, 6, 5708–5717.
Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z. R.; Watanabe, F.; Biris, A. S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227.
Wilson, A. T.; Calvin, M. The Photosynthetic cycle. CO2 dependent transients. J. Am. Chem. Soc. 1955, 77, 5948–5957.
Walker, D. A. Three phases of chloroplast research. Nature 1970, 226, 1204–1208.
Lin, M. T.; Occhialini, A.; Andralojc, P. J.; Parry, M. A. J.; Hanson, M. R. A faster rubisco with potential to increase photosynthesis in crops. Nature 2014, 513, 547–550.
Wong, S. W.; Cowan, I. R.; Farquhar, G. D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426.
Kotchey, G. P.; Hasan, S. A.; Kapralov, A. A.; Ha, S. H.; Kim, K.; Shvedova, A. A.; Kagan, V. E.; Star, A. A natural vanishing act: The enzyme-catalyzed degradation of carbon nanomaterials. Acc. Chem. Res. 2012, 45, 1770–1781.
Allen, B. L.; Kotchey, G. P.; Chen, Y. N.; Yanamala, N. V. K.; Klein-Seetharaman, J.; Kagan, V. E.; Star, A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205.
Went, F. W.; Thimann, K. V. Phytohormones; The Macmillan Company: New York, 1937.
Nurunnabi, M.; Khatun, Z.; Huh, K. M.; Park, S. Y.; Lee, D. Y.; Cho, K. J.; Lee, Y. K. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 2013, 7, 6858–6867.
Acknowledgements
This work is supported by the National MCF Energy R&D Program (No. 2018YFE0306105), the National Natural Science Foundation of China (Nos. 51725204, 51572179, 21771132, and 21471106), the Natural Science Foundation of Jiangsu Province (No. BK20161216), Collaborative Innovation Center of Suzhou Nano Science & Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the 111 Project.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, H., Huang, J., Liu, Y. et al. Enhanced RuBisCO activity and promoted dicotyledons growth with degradable carbon dots. Nano Res. 12, 1585–1593 (2019). https://doi.org/10.1007/s12274-019-2397-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2397-5