Abstract
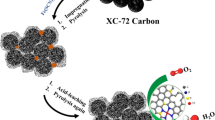
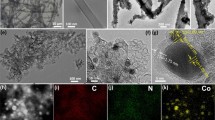
The capability of electrocatalytic reduction of carbon dioxide (CO2) using nitrogen (N)-doped carbon strongly depends on the N-doping level and their types. In this work, we developed a strategy to generate mesoporous N-doped carbon frameworks with tunable configurations and contents of N dopants, by using a secondary doping process via the treatment of N,N-dimethylformamide (DMF) solvent. The obtained mesoporous N-doped carbon (denoted as MNC-D) served as an efficient electrocatalyst for electroreduction of CO2 to CO. A high Faradaic efficiency of ∼ 92% and a partial current density for CO of −6.8 mA·cm−2 were achieved at a potential of −0.58 V vs. RHE. Electrochemical analyses further revealed that the active sites within the N-doped carbon catalysts were the pyridinic N and defects generated by the DMF treatment, which enhanced the activation and adsorption CO2 molecules. Our study suggests a new approach to develop efficient carbon-based catalysts for potential scalable CO2RR to fuels and chemicals.

Similar content being viewed by others
References
Zhu, D. D.; Liu, J. L.; Qiao, S. Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 2016, 28, 3423–3452.
Vasileff, A.; Zheng, Y.; Qiao, S. Z. Carbon solving carbon’s problems: Recent progress of nanostructured carbon-based catalysts for the electrochemical reduction of CO2. Adv. Energy Mater. 2017, 7, 1700759.
Wu, J. H.; Huang, Y.; Ye, W.; Li, Y. G. CO2 reduction: From the electrochemical to photochemical approach. Adv. Sci. 2017, 4, 1700194.
Kuang, M.; Han, P.; Huang, L. S.; Cao, N.; Qian, L. P.; Zheng, G. F. Electronic tuning of Co, Ni-based nanostructured (hydr)oxides for aqueous electrocatalysis. Adv. Funct. Mater. 2018, 28, 1804886.
Xie, M. S.; Xia, B. Y.; Li, Y. W.; Yan, Y.; Yang, Y. H.; Sun, Q.; Chan, S. H.; Fisher, A.; Wang, X. Amino acid modified copper electrodes for the enhanced selective electroreduction of carbon dioxide towards hydrocarbons. Energy Environ. Sci. 2016, 9, 1687–1695.
Zheng, T. T.; Jiang, K.; Wang, H. T. Recent advances in electrochemical CO2-to-CO conversion on heterogeneous catalysts. Adv. Mater. 2018, 30, 1802066.
Zhang, X.; Wu, Z. S.; Zhang, X.; Li, L. W.; Li, Y. Y.; Xu, H. M.; Li, X. X.; Yu, X. L.; Zhang, Z. S.; Liang, Y. Y. et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675.
Zhu, W. L.; Michalsky, R.; Metin, Ö.; Lv, H. F.; Guo, S. J.; Wright, C. J.; Sun, X. L.; Peterson, A. A.; Sun, S. H. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836.
Kim, C.; Jeon, H. S.; Eom, T.; Jee, M. S.; Kim, H.; Friend, C. M.; Min, B. K; Hwang, Y. J. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850.
Jiang, B.; Zhang, X. G.; Jiang, K.; Wu, D. Y.; Cai, W. B. Boosting formate production in electrocatalytic CO2 reduction over wide potential window on Pd surfaces. J. Am. Chem. Soc. 2018, 140, 2880–2889.
Rosen, J.; Hutchings, G. S.; Lu, Q.; Forest, R. V.; Moore, A.; Jiao, F. Electrodeposited Zn dendrites with enhanced CO selectivity for electro-catalytic CO2 reduction. ACS Catal. 2015, 5, 4586–4591.
Reske, R.; Mistry, H.; Behafarid, F.; Cuenya, B. R.; Strasser, P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986.
Gu, Z. X.; Yang, N.; Han, P.; Kuang, M.; Mei, B. B.; Jiang, Z.; Zhong, J.; Li, L.; Zheng, G. F. Oxygen vacancy tuning toward efficient electrocatalytic CO2 reduction to C2H4. Small Methods 2019, 3, 1800449.
Yang, H.; Han, N.; Deng, J.; Wu, J. H.; Wang, Y.; Hu, Y. P.; Ding, P.; Li, Y. F.; Li, Y. G.; Lu, J. Selective CO2 reduction on 2D mesoporous Bi nanosheets, Adv. Energy Mater. 2018, 8, 1801536.
Kim, D.; Resasco J.; Yu, Y.; Asiri, A. M.; Yang, P. D. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948.
Geng, Z. G.; Kong, X. D.; Chen, W. W.; Su, H. Y.; Liu, Y.; Cai, F.; Wang, G. X.; Zeng, J. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO. Angew. Chem., Int. Ed. 2018, 57, 6054–6059.
Hoang, T. T. H.; Verma, S.; Ma, S. C.; Fister, T. T.; Timoshenko, J.; Frenkel, A. I.; Kenis, P. J. A.; Gewirth, A. A. Nanoporous copper-silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797.
Zhao, Y.; Liang, J. J.; Wang, C. Y.; Ma, J. M.; Wallace, G. G. Tunable and efficient tin modified nitrogen-doped carbon nanofibers for electrochemical reduction of aqueous carbon dioxide. Adv. Energy Mater. 2018, 8, 1702524.
Xie, J. F.; Zhao, X. T.; Wu, M. X.; Li, Q. H.; Wang, Y. B; Yao, J. N. Metal-free fluorine-doped carbon electrocatalyst for CO2 reduction outcompeting hydrogen evolution. Small 2018, 57, 9640–9644.
Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B. A.; Haasch, R.; Abiade, J.; Yarin, A. L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 2819.
Liu, Y. M.; Chen, S.; Quan, X.; Yu, H. T. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636.
Sharma, P. P.; Wu, J. J.; Yadav, R. M.; Liu, M. J.; Wright, C. J.; Tiwary, C. S.; Yakobson, B. I.; Lou, J.; Ajayan, P. M.; Zhou, X. D. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: On the understanding of defects, defect density, and selectivity. Angew. Chem., Int. Ed. 2015, 54, 13701–13705.
Chai, G. L.; Guo, Z. X. Highly effective sites and selectivity of nitrogen-doped graphene/CNT catalysts for CO2 electrochemical reduction. Chem. Sci. 2016, 7, 1268–1275.
Han, P.; Yu, X. M.; Yuan, D.; Kuang, M.; Wang, Y. F.; Al-Enizi, A. M.; Zheng, G. F. Defective graphene for electrocatalytic CO2 reduction. J. Colloid Interface Sci. 2019, 534, 332–337.
Tao, L.; Qiao, M.; Jin, R.; Li, Y.; Xiao, Z. H.; Wang, Y. Q.; Zhang, N. N.; Xie, C.; He, Q. G.; Jiang, D. C. et al. Bridging the surface charge and catalytic activity of a defective carbon electrocatalyst. Angew. Chem., Int. Ed. 2019, 58, 1019–1024.
Wu, J. J.; Yadav, R. M.; Liu, M. J.; Sharma, P. P.; Tiwary, C. S.; Ma, L. L.; Zou, X. L.; Zhou, X. D.; Yakobson, B. I.; Lou, J. et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes. ACS Nano 2015, 9, 5364–5371.
Liu, K. H.; Zhong, H. X.; Yang, X. Y.; Bao, D.; Meng, F. L.; Yan, J. M.; Zhang, X. B. Composition-tunable synthesis of “clean” syngas via a one-step synthesis of metal-free pyridinic-N-enriched self-supported CNTs: The synergy of electrocatalyst pyrolysis temperature and potential. Green Chem. 2017, 19, 4284–4288.
Wu, J. J.; Liu, M. J.; Sharma, P. P.; Yadav, R. M.; Ma, L. L.; Yang, Y. C.; Zou, X. L.; Zhou, X. D.; Vajtai, R.; Yakobson, B. I. et al. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam. Nano Lett. 2016, 16, 466–470.
Cui, X. Q.; Pan, Z. Y.; Zhang, L. J.; Peng, H. S.; Zheng, G. F. Selective etching of nitrogen-doped carbon by steam for enhanced electrochemical CO2 reduction. Adv. Energy Mater. 2017, 7, 1701456.
Wang, H.; Jia, J.; Song, P. F.; Wang, Q.; Li, D. B.; Min, S. X.; Qian, C. X.; Wang, L.; Li, Y. F.; Ma, C. et al. Efficient electrocatalytic reduction of CO2 by nitrogen-doped nanoporous carbon/carbon nanotube membranes: A step towards the electrochemical CO2 refinery. Angew. Chem. 2017, 129, 7955–7960.
Liang, Z. B.; Qu, C.; Guo, W. H.; Zou, R. Q.; Xu, Q. Pristine metal-organic frameworks and their composites for energy storage and conversion. Adv. Mater. 2018, 30, 1702891.
Zheng, F. C.; Yang, Y.; Chen, Q. W. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Commun. 2014, 5, 5261.
Torad, N. L.; Hu, M.; Kamachi, Y.; Takai, K.; Imura, M.; Naito, M.; Yamauchi, Y. Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chem. Commun. 2013, 49, 2521–2523.
Yu, H. Y.; Fisher, A.; Cheng, D. J.; Cao, D. P. Cu,N-codoped hierarchical porous carbons as electrocatalysts for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 21431–21439.
Lai, Q. X.; Zhao, Y. X.; Liang, Y. Y.; He, J. P.; Chen, J. H. In situ confinement pyrolysis transformation of ZIF-8 to nitrogen-enriched meso-microporous carbon frameworks for oxygen reduction. Adv. Funct. Mater. 2016, 26, 8334–8344.
Wu, J. J.; Ma, S. C.; Sun, J.; Gold, J. I.; Tiwary, C. S.; Kim, B.; Zhu, L. Y.; Chopra, N.; Odeh, I. N.; Vajtai, R. et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 2016, 7, 13869.
Jiang, R.; Li, L.; Sheng, T.; Hu, G. F.; Chen, Y. G.; Wang, L. Y. Edge-site engineering of atomically dispersed Fe-N4 by selective C–N bond cleavage for enhanced oxygen reduction reaction activities. J. Am. Chem. Soc. 2018, 140, 11594–11598.
Hou, Y.; Wen, Z. H.; Cui, S. M.; Ci, S. Q.; Mao, S.; Chen J. H. An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv. Funct. Mater. 2015, 25, 872–882.
Varela, A. S.; Sahraie, N. R.; Steinberg, J.; Ju, W.; Oh, H. S.; Strasser, P. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons. Angew. Chem., Int. Ed. 2015, 54, 10758–10762.
Niu, F. E.; Yang, J.; Wang, N. N.; Zhang, D. P.; Fan, W. L.; Yang, J.; Qian, Y. T. MoSe2-covered N,P-doped carbon nanosheets as a long-life and high-rate anode material for sodium-ion batteries. Adv. Funct. Mater. 2017, 27, 1700522.
Kuang, M.; Wang, Q. H.; Han, P.; Zheng, G. F. Cu, Co-embedded N-enriched mesoporous carbon for efficient oxygen reduction and hydrogen evolution reactions. Adv. Energy Mater. 2017, 7, 1700193.
White, R. J.; Yoshizawa, N.; Antonietti, M.; Titirici, M. M. A sustainable synthesis of nitrogen-doped carbon aerogels. Green Chem. 2011, 13, 2428–2434.
Baccile, N.; Laurent, G.; Coelho, C.; Babonneau, F.; Zhao, L.; Titirici, M. M. Structural insights on nitrogen-containing hydrothermal carbon using solid-state magic angle spinning 13C and 15N nuclear magnetic resonance. J. Phys. Chem. C 2011, 115, 8976–8982.
Gong, J.; Antonietti, M.; Yuan, J. Y. Poly(ionic liquid)-derived carbon with site-specific N-doping and biphasic heterojunction for enhanced CO2 capture and sensing. Angew. Chem. 2017, 129, 7665–7671.
Verdaguer-Casadevall, A.; Li, C. W.; Johansson, T. P.; Scott, S. B.; McKeown, J. T.; Kumar, M.; Stephens I. E. L.; Kanan, M. W.; Chorkendorff, I. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 2015, 137, 9808–9811.
Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B. A.; Haasch, R.; Abiade, J.; Yarin, A. L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 2819.
Wentrup, C.; Winter, H. W. Isolation of diazacycloheptatetraenes from thermal nitrene-nitrene rearrangements. J. Am. Chem. Soc. 1980, 102, 6159–6161.
Zheng, F. C.; Yang, Y.; Chen, Q. W. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Commun. 2014, 5, 5261.
Acknowledgements
We thank the following funding agencies for supporting this work: the National Key Research and Development Program of China (Nos. 2017YFA0206901 and 2018YFA0209401), the National Natural Science Foundation of China (No. 21773036), the Science and Technology Commission of Shanghai Municipality (Nos. 17JC1402000 and 19XD1420400), and the Innovation Program of Shanghai Municipal Education Commission (No. 2019-01-07-00-07-E00045).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Kuang, M., Guan, A., Gu, Z. et al. Enhanced N-doping in mesoporous carbon for efficient electrocatalytic CO2 conversion. Nano Res. 12, 2324–2329 (2019). https://doi.org/10.1007/s12274-019-2396-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2396-6