Abstract
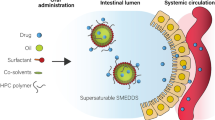
A supersaturating self-emulsifying drug delivery system (S-SEDDS) was prepared and evaluated for enhanced dissolution of celecoxib (CXB), a poorly water-soluble drug. The selected CXB-dissolved SEDDS formulation consisting 10 % Capryol 90 (oil), 45 % Tween 20 (surfactant), and 45 % Tetraglycol (cosurfactant) had the characteristics of small droplet size and great solubility as 208 nm and 556.7 mg/mL in average, respectively. CXB dissolution from SEDDS in simulated gastric fluid was increased to about 20 % for the initial period of 5 min, but decreased to a half level as time elapsed. Thus, precipitation inhibitors were screened to stabilize the supersaturation. The stabilizing effect of Soluplus, an amphiphilic copolymer, was concentration-dependent, revealing the greatest dissolution of approximately 90 % level with delayed drug crystallization by the addition of the copolymer. CXB dissolution from S-SEDDS was pH-independent. We concluded that S-SEDDS formulation would be very useful in the future for developing oral delivery product of poorly water-soluble drugs.








Similar content being viewed by others
References
Amidon, G.L., H. Lennernas, V.P. Shah, and J.R. Crison. 1995. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharmaceutical Research 12: 413–420.
Augustijns, P., and M.E. Brewster. 2012. Supersaturating drug delivery systems: Fast is not necessarily good enough. Journal of Pharmaceutical Sciences 101: 7–9.
BASF, Soluplus—Technical Information. 2010. The BASF Chemical Company Pharma Ingredients & Services, Limburgerhof, Germany, 3.
Brouwers, J., M.E. Brewster, and P. Augustijns. 2009. Supersaturating drug delivery systems: The answer to solubility-limited oral bioavailability? Journal of Pharmaceutical Sciences 98: 2549–2572.
Castile, J.D., K.M.G. Taylor, and G. Buckton. 2001. The influence of incubation temperature and surfactant concentration of the interaction between dimyristoylphophatidylcholine liposomes and poloxamer surfactant. International Journal of Pharmaceutics 221: 197–209.
Clemett, D., and K.L. Goa. 2000. Celecoxib: A review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs 59: 957–980.
Connor, E.P. 2003. Physicians’ desk reference, 57th ed, 2589–2593. Montvale: Medical Economics Company.
Constantinides, P.P. 1995. Lipid microemulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects. Pharmaceutical Research 12: 1561–1572.
Dai, W.G., L.C. Dong, S. Li, and Z. Deng. 2008. Combination of pluronic/vitamin E TPGS as a potential inhibitor of drug precipitation. International Journal of Pharmaceutics 35: 31–37.
Gao, P., B.D. Rush, W.P. Pfund, T. Huang, J.M. Bauer, W. Morozowich, M.S. Kuo, and M.J. Hageman. 2003. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. Journal of Pharmaceutical Sciences 92: 2386–2398.
Gao, P., M.E. Guyton, T. Huang, J.M. Bauer, K.J. Stefanski, and Q. Lu. 2004. Enhanced oral bioavailability of a poorly water soluble drug PNU-91325 by supersaturable formulations. Drug Development and Industrial Pharmacy 30: 221–229.
Gupta, P., V.K. Kakumanu, and A.K. Bansal. 2004. Stability and solubility of celecoxib–PVP amorphous dispersions: A molecular perspective. Pharmaceutical Research 21: 1762–1769.
Guzmán, H.R., M. Tawa, Z. Zhang, P. Ratanabanangkoon, P. Shaw, C.R. Gardner, H. Chen, J.P. Moreau, O. Almarsson, and J.F. Remenar. 2007. Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. Journal of Pharmaceutical Sciences 96: 2686–2702.
Joshi, M., and V. Patravale. 2008. Nanostructured lipid carrier (NLC) based gel of celecoxib. International Journal of Pharmaceutics 346: 123–132.
Kang, M.J., H.S. Kim, H.S. Jeon, J.H. Park, B.S. Lee, B.K. Ahn, K.Y. Moon, and Y.W. Choi. 2012. In situ intestinal permeability and in vivo absorption characteristics of olmesartan medoxomil in self-microemulsifying drug delivery system. Drug Development and Industrial Pharmacy 38: 587–596.
Khoo, S.M., A.J. Humberstone, C.J.H. Porter, G.A. Edwards, and W.N. Charman. 1998. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. International Journal of Pharmaceutics 167: 155–164.
Lee, S.G., J.H. Jeong, S.R. Kim, K.M. Lee, B.K. Ahn, M.H. Kang, and Y.W Choi. 2012a. Topical formulation of retinyl retinoate employing nanostructured lipid carriers. Journal of Pharmaceutical Investigation. doi:10.1007/s40005-012-0036-1.
Lee, Y.H., E.H. Jung, H.J. Kim, J.H. Yoon, D.D. Kim, and Y.J. Jung. 2012. Preparation and in vitro evaluation of celecoxib–amino acid conjugates as a colon specific prodrug. Journal of Pharmaceutical Investigation 42: 115–120.
Linn, M., E.M. Collnot, D. Djuric, K. Hempel, E. Fabian, K. Kolter, and C.M. Lehr. 2012. Soluplus as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. European Journal of Pharmaceutical Sciences 45: 336–343.
Lu, G.W., M. Hawley, M. Smith, B.M. Geiger, and W. Pfund. 2006. Characterization of a novel polymorphic form of celecoxib. Journal of Pharmaceutical Sciences 95: 305–317.
Morgen, M., C. Bloom, R. Beyerinck, A. Bello, W. Song, K. Wilkinson, R. Steenwyk, and S. Shambilin. 2012. Polymeric nanoparticles for increased oral bioavailability and rapid absorption using celecoxib as a model of a low-solubility, high-permeability drug. Pharmaceutical Research 29: 427–440.
Nagarsenker, M.S., and M.S. Joshi. 2005. Celecoxib–cyclodextrin systems: Characterization and evaluation of in vitro and in vivo advantage. Drug Development and Industrial Pharmacy 31: 169–178.
Nan, Z., G. Lijun, W. Tao, and Q. Dongqin. 2012. Evaluation of carbamazepine (CBZ) supersaturatable self microemulsifying (S-SMEDDS) formulation in vitro and in vivo. Iranian Journal of Phamaceutical Research 11: 257–264.
Paulson, S.K., M.B. Vaughn, S.M. Jessen, Y. Lawal, C.J. Gresk, B. Yan, T.J. Maziasz, C.S. Cook, and A. Karim. 2001. Pharmacokinetics of celecoxib after oral administration in dogs and humans: Effect of food and site of absorption. Journal of Pharmacology and Experimental Therapeutics 297: 638–645.
Pouton, C.W. 2000. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemusifying’ drug delivery systems. European Journal of Pharmaceutical Sciences 11: S93–S98.
Raghavan, S.L., A. Trividic, A.F. Davis, and J. Hadgraft. 2001. Crystallization of hydrocortisone acetate: Influence of polymers. International Journal of Pharmaceutics 212: 213–221.
Rahman, M.A., Z. Iqbal, and A. Hussian. 2012. Formulation of optimization and in vitro characterization of sertraline loaded self-nanoemulsifying drug delivery system (SNEDDS) for oral administration. Journal of Pharmaceutical Investigation 42: 191–202.
Remenar, J.F., M.L. Peterson, P.W. Stephenus, Z. Zhang, Y. Zimenkov, and M.B. Hickey. 2007. Celecoxib nicotinamide dissociation: Using excipients to capture the cocrystal’s potential. Molecular Pharmaceutics 4: 386–400.
Shah, N.H., M.T. Carvajal, C.I. Pater, M.H. Infeld, and A.W. Malick. 1994. Self-emsulsifying drug delivery systems with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Journal of Pharmaceutical Investigation 106: 15–23.
Sharma, A., and C.P. Jain. 2010. Preparation and characterization of solid dispersions of carvedilol with PVP K 30. Research in Pharmaceutical Sciences 5: 49–56.
Shen, H., and M. Zhong. 2006. Preparation and evaluation and function of self micromemulsifying drug delivery system (SMEDDS) contacting atorvastatin. Journal of Pharmacy and Pharmacology 56: 1183–1191.
Subramanian, N., S. Ray, S.K. Ghosal, R. Bhadra, and S.P. Moulik. 2004. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biological and Pharmaceutical Bulletin 27: 1993–1999.
Woodle, M.C., M.S. Newman, and F.J. Martin. 1992. Liposome leakage and blood circulation: Comparison of adsorbed block copolymers with covalent attachment of PEG. International Journal of Pharmaceutics 88: 327–334.
Acknowledgments
This research was supported by the Chung-Ang University Research Scholarship Grants in 2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Woo Heon Song and Jong Hyeok Park contributed equally to this work.
Rights and permissions
About this article
Cite this article
Song, W.H., Park, J.H., Yeom, D.W. et al. Enhanced dissolution of celecoxib by supersaturating self-emulsifying drug delivery system (S-SEDDS) formulation. Arch. Pharm. Res. 36, 69–78 (2013). https://doi.org/10.1007/s12272-013-0011-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0011-z