Abstract
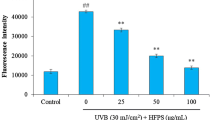
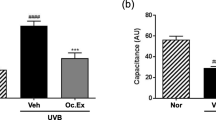
Ultraviolet (UV) irradiation induces skin photoaging by generating reactive oxygen species (ROS). ROS caused by UV-irradiation results in loss of skin cells and degradation of extracellular matrix. A number of antioxidants have been chemically synthesized or naturally extracted to prevent ROS-mediated skin photoaging. In our previous work, silkworm hemolymph extract (SHEX) was prepared, and its antioxidant activity was tested by free radical-scavenging assay. This study assessed the protective effects of SHEX on UV-induced photoaging of human immortalized keratinocytes (HaCaT). UVA (365 nm)-induced ROS generation was inhibited by supplementation of silkworm hemolymph (SH). Treatment with SHEX prepared by boiling SH inhibited death of HaCaT cells caused by UVB (315 nm) and UVA irradiation in a dose-dependent manner. Seven fractions were obtained by separating SHEX by gel permeation chromatography and the antioxidant activity of the fractions was examined. The fraction showing the highest protective efficacy on UV-induced cell damage corresponded to the lutein-containing fraction isolated in our previous study. Moreover, the SHEX fraction suppressed the expression of MMP-1 (matrix metalloproteinase-1), a matrix-degrading enzyme, suggesting that the active constituent of SHEX has the potential to inhibit skin photoaging. These results suggest that SHEX can be developed as a dietary and cosmetic supplement for prevention of skin photoaging.
Similar content being viewed by others
References
Fisher, G. J., S. Kang, J. Varani, Z. Bata-Csorgo, Y. Wan, S. Datta, and J. J. Voorhees (2002) Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 138: 1462–1470.
Rittié, L. and G. J. Fisher (2002) UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 1: 705–720.
Afaq, F. (2011) Natural agents: Cellular and molecular mechanisms of photoprotection. Arch. Biochem. Biophys. 508: 144–151.
Aoki-Yoshida, A., R. Aoki, and Y. Takayama (2013) Protective effect of pyruvate against UVB-induced damage in HaCaT human keratinocytes. J. Biosci. Bioeng. 115: 442–448.
Rezvani, H. R., F. Mazurier, M. Cario-André, C. Pain, C. Ged, A. Taïeb, and H. de Verneuil (2006) Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J. Biol. Chem. 281: 17999–18007.
Ichihashi, M., M. Ueda, A. Budiyanto, T. Bito, M. Oka, M. Fukunaga, K. Tsuru, and T. Horikawa (2003) UV-induced skin damage. Toxicol. 189: 21–39.
Agar, N. S., G. M. Halliday, R. S. Barnetson, H. N. Ananthaswamy, M. Wheeler, and A. M. Jones (2004) The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA. 101: 4954–4959.
Mitchell, D. (2006) Revisiting the photochemistry of solar UVA in human skin. Proc. Natl. Acad. Sci. USA. 103: 13567–13568.
Birch-Machin, M. A. and H. Swalwell (2009) How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis 25: 101–107.
Battie, C., S. Jitsukawa, F. Bernerd, S. Del Bino, C. Marionnet, and M. Verschoore (2014) New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 23: 7–12.
Calò, R. and L. Marabini (2014) Protective effect of Vaccinium myrtillus extract against UVA-and UVB-induced damage in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B 132: 27–35.
Pupe, A., P. De Haes, L. Rhodes, M. Garmyn, R. Moison, G. B. van Henegouwen, and H. Degreef (2002) Eicosapentaenoic acid, a n-3 polyunsaturated fatty acid differentially modulates TNF-a, IL-1a, IL-6 and PGE 2 expression in UVB-irradiated human keratinocytes. J. Invest. Dermatol. 118: 692–698.
Pillai, S., C. Oresajo, and J. Hayward (2005) Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation? induced matrix degradation–a review. Int. J. Cosmet. Sci. 27: 17–34.
Visse, R. and H. Nagase (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ. Res. 92: 827–839.
Brennan, M., H. Bhatti, K. C. Nerusu, N. Bhagavathula, S. Kang, G. J. Fisher, J. Varani, and J. J. Voorhees (2003) Matrix metalloproteinase? 1 is the major collagenolytic enzyme responsible for collagen damage in UV?irradiated human skin. Photochem. Photobiol. 78: 43–48.
Quan, T., Z. Qin, W. Xia, Y. Shao, J. J. Voorhees, and G. J. Fisher (2009) Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 14: 20–24.
Rice-Evans, C., N. Miller, and G. Paganga (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci. 2: 152–159.
Kähkönen, M. P., A. I. Hopia, H. J. Vuorela, J.-P. Rauha, K. Pihlaja, T. S. Kujala, and M. Heinonen (1999) Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47: 3954–3962.
Katiyar, S. K., F. Afaq, A. Perez, and H. Mukhtar (2001) Green tea polyphenol (–)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 22: 287–294.
Xia, J., X. Song, Z. Bi, W. Chu, and Y. Wan (2005) UV-induced NF-?B activation and expression of IL-6 is attenuated by (-)-epigallocatechin-3-gallate in cultured human keratinocytes in vitro. Int. J. Mol. Med. 16: 943–950.
Jeon, H., J. Kim, W. Kim, and S. Lee (2009) Effects of oral epigallocatechin gallate supplementation on the minimal erythema dose and UV-induced skin damage. Skin Pharmacol. Physiol. 22: 137–141.
Frémont, L. (2000) Biological effects of resveratrol. Life Sci. 66: 663–673.
Afaq, F., V. M. Adhami, and N. Ahmad (2003) Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 186: 28–37.
Iannone, A., C. Rota, S. Bergamini, A. Tomasi, and L. M. Canfield (1998) Antioxidant activity of carotenoids: An electron?spin resonance study on ß?carotene and lutein interaction with free radicals generated in a chemical system. J. Biochem. Mol. Toxicol. 12: 299–304.
Cesarini, J., L. Michel, J. Maurette, H. Adhoute, and M. Bejot (2003) Immediate effects of UV radiation on the skin: modification by an antioxidant complex containing carotenoids. Photodermatol. Photoimmunol. Photomed. 19: 182–189.
Sies, H. and W. Stahl (2004) Carotenoids and UV protection. Photochem. Photobiol. Sci. 3: 749–752.
Perry, A., H. Rasmussen, and E. J. Johnson (2009) Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compost. Anal. 22: 9–15.
Pintea, A., D. O. Rugina, R. Pop, A. Bunea, and C. Socaciu (2011) Xanthophylls protect against induced oxidation in cultured human retinal pigment epithelial cells. J. Food Compost. 24: 830–836.
Junghans, A., H. Sies, and W. Stahl (2001) Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 391: 160–164.
Ma, L., H.-L. Dou, Y.-Q. Wu, Y.-M. Huang, Y.-B. Huang, X.-R. Xu, Z.-Y. Zou, and X.-M. Lin (2012) Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 107: 350–359.
Rhee, W. J. and T. H. Park (2000) Silkworm hemolymph inhibits baculovirus-induced insect cell apoptosis. Biochem. Biophys. Res. Commun. 271: 186–190.
Choi, S. S., W. J. Rhee, and T. H. Park (2002) Inhibition of human cell apoptosis by silkworm hemolymph. Biotechnol. Prog. 18: 874–878.
Rhee, W. J., E. J. Kim, and T. H. Park (2002) Silkworm hemolymph as a potent inhibitor of apoptosis in Sf9 cells. Biochem. Biophys. Res. Commun. 295: 779–783.
Kim, E. J., W. J. Rhee, and T. H. Park (2001) Isolation and characterization of an apoptosis-inhibiting component from the hemolymph of Bombyx mori. Biochem. Biophys. Res. Commun. 285: 224–228.
Kim, E. J., H. J. Park, and T. H. Park (2003) Inhibition of apoptosis by recombinant 30K protein originating from silkworm hemolymph. Biochem. Biophys. Res. Commun. 308: 523–528.
Kim, E. J., W. J. Rhee, and T. H. Park (2004) Inhibition of apoptosis by a Bombyx mori gene. Biotechnol. Prog. 20: 324–329.
Choi, S. S., W. J. Rhee, E. J. Kim, and T. H. Park (2006) Enhancement of recombinant protein production in Chinese hamster ovary cells through anti-apoptosis engineering using 30Kc6 gene. Biotechnol. Bioeng. 95: 459–467.
Koo, T. Y., J. H. Park, H. H. Park, and T. H. Park (2009) Beneficial effect of 30Kc6 gene expreßsion on production of recombinant interferon-ß in serum-free suspension culture of CHO cells. Proc. Biochem. 44: 146–153.
Park, J. H., H. H. Park, and T. H. Park (2010) Cellular engineering for the high-level production of recombinant proteins in mammalian cell systems. Kor. J. Chem. Eng. 27: 1042–1048.
Wang, Z., J. H. Park, H. H. Park, W. Tan, and T. H. Park (2010) Enhancement of therapeutic monoclonal antibody production in CHO cells using 30Kc6 gene. Proc. Biochem. 45: 1852–1856.
Choi, S. S. and T. H. Park (2013) Antioxidant effect of proteinfree silkworm hemolymph extract in mitochondrial membrane potential. Food Sci. Biotechnol. 22: 233–239.
Mangels, A. R., J. M. Holden, G. R. Beecher, M. R. Forman, and E. Lanza (1993) Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet. Assoc. 93: 284–296.
Tabunoki, H., S. Higurashi, O. Ninagi, H. Fujii, Y. Banno, M. Nozaki, M. Kitajima, N. Miura, S. Atsumi, and K. Tsuchida (2004) A carotenoid?binding protein (CBP) plays a crucial role in cocoon pigmentation of silkworm (Bombyx mori) larvae. FEBS Lett. 567: 175–178.
Prommuak, C., W. De-Eknamkul, and A. Shotipruk (2008) Extraction of flavonoids and carotenoids from Thai silk waste and antioxidant activity of extracts. Sep. Purif. Technol. 62: 444–448.
Aimjongjun, S., M. Sutheerawattananonda, and N. Limpeanchob (2013) Silk lutein extract and its combination with vitamin E reduce UVB-mediated oxidative damage to retinal pigment epithelial cells. J. Photochem. Photobiol. B 124: 34–41.
Wrona, M., M. Rózanowska, and T. Sarna (2004) Zeaxanthin in combination with ascorbic acid or a-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med. 36: 1094–1101.
Ryu, K. S., H. S. Lee, S. H. Chung, and P. D. Kang (1997) An activity of lowering blood-glucose levels according to preparative condition of silkworm powder. Kor. J. Seric. Sci. 39: 79–85.
Ryu, K. S., H. S. Lee, and I. Kim (2002) Effects and mechanisms of silkworm powder as a blood glucose-lowerinly agent. Int. J. Indust. Entomol. 4: 93–100.
Ryu, K. S., H. S. Lee, K. Y. Kim, M. J. Kim, and P. D. Kang (2013) Heat stability and glucose-lowering effect of 1-deoxynojirimycin from silkworm (Bombyx mori) extract powder. Int. J. Indust. Entomol. 27: 277–281.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ju Hyun Park and Ji Eun Lee contributed equally to this study.
Rights and permissions
About this article
Cite this article
Park, J.H., Lee, J.E., Choi, S.S. et al. Protective effects of silkworm hemolymph extract and its fractions on UV-induced photoaging. Biotechnol Bioproc E 22, 37–44 (2017). https://doi.org/10.1007/s12257-016-0588-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-016-0588-4