Abstract
Purpose
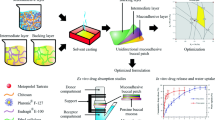
Therapeutic efficacy of zolmitriptan in oral therapy is primarily limited by the biopharmaceutical issues. The objective of this study is to design and optimize chitosan-based buccal bioadhesive system for the effective delivery of zolmitriptan in the treatment of migraine.
Methods
Factorial design (32) is constructed and conducted in a fully randomized manner to study all nine possible experimental runs. The films were prepared by solvent casting method by varying the content of chitosan (X1) and polyvinyl alcohol (X2). The effect of these two independent variables on swelling index (Y1), percent drug release in 15 min (Y2) and 5 h (Y3), and mucoadhesive strength (Y4) of prepared films was evaluated.
Results
The physical and chemical characteristics displayed by the prepared films (F1–F9) were found to be optimal. It was observed that the factor X1 has positive and X2 has negative effect on response Y1. In contrast, factor X1 showed negative effects on drug release at both time intervals (15 min and 5 h) while X2 displayed positive responses for these variables (Y2 and Y3). However, the mucoadhesion increased with an increase in factor X1 and decreased when the factor X2 was increased. Indeed, the desirable characteristics exhibited by the film F7 are ideal for buccal application. Greater flux (63.93 ± 12.51 μg/cm2/h) demonstrated in ex vivo studies substantiated the potential of optimized film to effectively deliver zolmitriptan across the buccal membrane.
Conclusions
This study concludes that the chitosan-based buccal film (F7) could be used in both prophylaxis and acute treatment of migraine, although need to be proved in vivo.





Similar content being viewed by others
References
Antonaci F, Dumitrache C, De Cillis I, Allena M. A review of current European treatment guidelines for migraine. J Headache Pain. 2010;11(1):13–9. https://doi.org/10.1007/s10194-009-0179-2.
Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619–29. https://doi.org/10.1523/JNEUROSCI.0373-15.2015.
Freitag FG. The cycle of migraine: patients’ quality of life during and between migraine attacks. Clin Ther. 2007;29(5):939–49. https://doi.org/10.1016/j.clinthera.2007.05.008.
Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol. 2012;15(Suppl 1):S15–22. https://doi.org/10.4103/0972-2327.99993.
Becker WJ. Acute migraine treatment in adults. Headache. 2015;55(6):778–93. https://doi.org/10.1111/head.12550.
Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5-HT1B/1D receptor agonists. Arch Neurol. 2002;59(7):1084–8. https://doi.org/10.1001/archneur.59.7.1084.
Antonaci F, Ghiotto N, Wu S, Pucci E, Costa A. Recent advances in migraine therapy. Spring. 2016;5(1):637. https://doi.org/10.1186/s40064-016-2211-8.
Dowson AJ, Charlesworth B. Review of zolmitriptan and its clinical applications in migraine. Expert Opin Pharmacother. 2002;3(7):993–1005. https://doi.org/10.1517/14656566.3.7.993.
Goadsby PJ, Boes CJ. Zolmitriptan: differences from sumatriptan. Curr Med Res Opin. 2001;17(Suppl 1):s46–50. https://doi.org/10.1185/0300799039117013.
Koteswari P, Sravanthi GP, Mounika M, Mohammed Rafi SK, Nirosha K. Formulation development and evaluation of zolmitriptan oral soluble films using 22 factorial designs. Int J Pharm Investig. 2016;6(4):201–6. https://doi.org/10.4103/2230-973X.195927.
Liu C, Fang L. Drug in adhesive patch of zolmitriptan: formulation and in vitro/in vivo correlation. AAPS PharmSciTech. 2015;16(6):1245–53. https://doi.org/10.1208/s12249-015-0303-3.
Shiledar RR, Tagalpallewar AA, Kokare CR. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr Polym. 2014;101:1234–42. https://doi.org/10.1016/j.carbpol.2013.10.072.
Kassem AA. Formulation approaches of triptans for management of migraine. Curr Drug Deliv. 2016;13(6):882–98. https://doi.org/10.2174/1567201813666160425112600.
Al-Dhubiab BE, Nair AB, Kumria R, Attimarad M, Harsha S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016;23(7):2154–62. https://doi.org/10.3109/10717544.2014.948644.
Scholz OA, Wolff A, Schumacher A, Giannola LI, Campisi G, Ciach T, et al. Drug delivery from the oral cavity: focus on a novel mechatronic delivery device. Drug Discov Today. 2008;13(5–6):247–53. https://doi.org/10.1016/j.drudis.2007.10.018.
Kumria R, Nair AB, Goomber G, Gupta S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016;23(2):471–8. https://doi.org/10.3109/10717544.2014.920058.
Montenegro-Nicolini M, Morales JO. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech. 2017;18(1):3–14. https://doi.org/10.1208/s12249-016-0525-z.
Gilhotra RM, Ikram M, Srivastava S, Gilhotra N. A clinical perspective on mucoadhesive buccal drug delivery systems. J Biomed Res. 2014;28(2):81–97. https://doi.org/10.7555/JBR.27.20120136.
Kumar S, Gupta SK. Natural polymers, gums and mucilages as excipients in drug delivery. Polim Med. 2012;42(3–4):191–7.
Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13(3):1133–74. https://doi.org/10.3390/md13031133.
Cheung RC, Ng TB, Wong JH, Chan WY. Chitosan: an update on potential biomedical and pharmaceutical applications. Mar Drugs. 2015;13(8):5156–86. https://doi.org/10.3390/md13085156.
Islam MA, Park TE, Reesor E, Cherukula K, Hasan A, Firdous J, et al. Mucoadhesive chitosan derivatives as novel drug carriers. Curr Pharm Des. 2015;21(29):4285–309. https://doi.org/10.2174/1381612821666150901103819.
Bernkop-Schnürch A, Dünnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81(3):463–9. https://doi.org/10.1016/j.ejpb.2012.04.007.
Duttagupta DS, Jadhav VM, Kadam VJ. Chitosan: a propitious biopolymer for drug delivery. Curr Drug Deliv. 2015;12(4):369–81. https://doi.org/10.2174/1567201812666150310151657.
Al-Dhubiab BE. Aripiprazole nanocrystal impregnated buccoadhesive films for schizophrenia. J Nanosci Nanotechnol. 2017;17(4):2345–52. https://doi.org/10.1166/jnn.2017.12588.
Kumria R, Nair AB, Al-Dhubiab BE. Loratidine buccal films for allergic rhinitis: development and evaluation. Drug Dev Ind Pharm. 2014;40(5):625–31. https://doi.org/10.3109/03639045.2014.884125.
Nair AB, Al-Ghannam AA, Al-Dhubiab BE, Hasan AA. Mucoadhesive film embedded with acyclovir loaded biopolymeric nanoparticles: in vitro studies. J Young Pharm. 2017;9(1):100–5. https://doi.org/10.5530/jyp.2017.9.19.
Nair AB, Kumria R, Harsha S, Attimarad M, Al-Dhubiab BE, Alhaider IA. In vitro techniques to evaluate buccal films. J Control Release. 2013;166(1):10–21. https://doi.org/10.1016/j.jconrel.2012.11.019.
Jug M, Hafner A, Lovrić J, Kregar ML, Pepić I, Vanić Ž, et al. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J Pharm Biomed Anal. 2018;147:350–66. https://doi.org/10.1016/j.jpba.2017.06.072.
Al-Dhubiab BE, Nair AB, Kumria R, Attimarad M, Harsha S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf B Biointerfaces. 2015;136:878–84. https://doi.org/10.1016/j.colsurfb.2015.10.045.
Nair AB, Sammeta SM, Vaka SRK, Murthy SN. A study on the effect of inorganic salts in transungual drug delivery of terbinafine. J Pharm Pharmacol. 2009;61(4):431–7. https://doi.org/10.1211/jpp.61.04.0003.
Shidhaye SS, Saindane NS, Sutar S, Kadam V. Mucoadhesive bilayered patches for administration of sumatriptan succinate. AAPS PharmSciTech. 2008;9(3):909–16. https://doi.org/10.1208/s12249-008-9125-x.
Nair A, Gupta R, Vasanti S. In vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm Dev Technol. 2007;12(6):621–5. https://doi.org/10.1080/10837450701563277.
Kumria R, Gupta V, Bansal S, Wadhwa J, Nair AB. Oral buccoadhesive films of ondansetron: development and evaluation. Int J Pharm Investig. 2013;3(2):112–8. https://doi.org/10.4103/2230-973X.114894.
Al-Dhubiab BE, Nair AB, Kumria R, Attimarad M, Harsha S. Development and evaluation of nebivolol hydrochloride nanocrystals impregnated buccal film. Farmacia. 2017; In press.
Pathak K, Sharma V, Akhtar N, Rastogi P. Localization of fluconazole in oral cavity by preferential coating of buccoadhesive tablet for treatment of oral thrush. Int J Pharm Investig. 2016;6(2):106–15. https://doi.org/10.4103/2230-973X.177826.
Panigrahi L, Pattnaik S, Ghosal SK. Design and characterization of mucoadhesive buccal patches of salbutamol sulphate. Acta Pol Pharm. 2004 Sep-Oct;61(5):351–60.
Acknowledgements
The authors acknowledge the Deanship of Scientific Research, King Faisal University (project no. 170078) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kumria, R., Al-Dhubiab, B.E., Shah, J. et al. Formulation and Evaluation of Chitosan-Based Buccal Bioadhesive Films of Zolmitriptan. J Pharm Innov 13, 133–143 (2018). https://doi.org/10.1007/s12247-018-9312-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9312-6