Abstract
Purpose
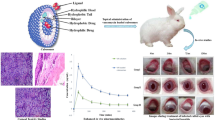
Poor intraocular bioavailability of valacyclovir (VACV) in the treatment of ocular viral infections is considered to be a challenging issue. Based on the fact that the polymethacrylic acid copolymers enhance the trans-corneal permeability of the encapsulated drug, the valacyclovir Eudragit® microspheres (VEM) were investigated.
Methods
The VEM prepared by nonaqueous emulsification solvent evaporation method was characterized by in vitro release study, release kinetics, X-ray diffraction (XRD), and the stability studies. The ocular irritancy, in vivo ocular pharmacokinetic parameters, pharmacokinetic/pharmacodynamic indices, simulation, and histopathology studies were performed in Wistar rats to ensure ocular tolerability and clinical effectiveness of the formulation.
Results
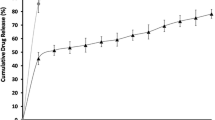
The in vitro drug release showed initial burst and followed Fickian (R square 0.9735, n value 0.1608) type of diffusion release mechanism. The XRD and stability studies showed favorable results. The Wistar rat eyes treated with VEM showed significant increase in ACV AUC (~2.55-fold) and Cmax (1.25-fold) in aqueous humor compared to VACV solution, and the delay in Tmax infers the sustained action of the VEM. The Cmax/MIC90, AUC0-24/MIC90, AUC above MIC90, and T above MIC90 were significantly higher in VEM group and thus indicates its clinical effectiveness. The aqueous humor concentration-time profile of ACV in VEM and VACV solution was simulated in every 5.2 and 2.5 h, respectively. The photomicrograph of VEM and VACV solution treated rat retina showed normal organization and cytoarchitecture.
Conclusions
The Eudragit®-based microspheres interact with the negatively charged conjunctival mucosa and anionic mucin of the tear film and thereby enhance intraocular VACV bioavailability.






Similar content being viewed by others
References
Tabbara KF, Balushi NA. Topical ganciclovir in the treatment of acute herpetic keratitis. Clin Opthalmol. 2010;4:905–12.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–60.
Hatanaka T, Haramura M, Fei YJ, Miyauchi S, Bridges CC, Ganapathy PS, et al. Transport of amino acid-based prodrugs by the NaC- and C1K-coupled amino acid transporter ATN0, C and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004;308:1138–47.
Clercq ED. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–33.
Kataragada S, Gunda S, HAriharan S, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008;359:15–24.
Das S, Suresh PK. Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomedicine: NBM. 2011;7:242–7. doi:10.1016/j.nano.2010.07.003.
Fatal E, Bochot A. Ocular delivery of nucleic acids: antisense oligonucleotides, aptamers and siRNA. Adv Drug Deliv Rev. 2006;58:1203–23.
Martinez-Sancho C, Herrero-Vanrell R, Negro S. Vitamin A palmitate and acyclovir biodegradable microspheres for intraocular sustained release. Int J Pharm. 2006;326:100–6.
Cochereau I, Diralson MC, Mousalatti H, Bouyer I, Ren L, Guvenisik N, et al. High-dose intravitreal ganciclovir in CMV retinitis. J Fr Ophtalmol. 2000;23:123–6.
Giordano GG, Refojo MF, Arroyo MH. Sustained delivery of retinoic acid from microsphere of biodegradable polymer in PVR. Invest Ophthalmol Vis Sci. 1993;34(9):2743–51.
Harper CA, Khoobehi B, Peyman G, Gebhardt BM, Dunlap WA. Bioavailability of microsphere-entrapped cyclosporine A in the cornea and aqueous of rabbits. Int Ophthalmol. 1993;17:337–40.
Behera BC, Sahoo SK, Dhal S, Barik BB, Gupta BK. Characterization of glipizide-loaded polymethacrylate microspheres prepared by an emulsion solvent evaporation method. Trop J Pharm Res. 2008;7(1):879–85.
Haznedar S, Dortunc B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm. 2001;269:131–40.
Khamanga SM, Parfitt N, Nyamuzhiwa T, Haidula H, Walker RB. The evaluation of Eudragit microcapsules manufactured by solvent evaporation using USP apparatus 1. Dissolut Techno. 2009;15–22.
Katara R, Majumdar DK. Eudragit RL 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloids Surf B: Biointerfaces. 2013;103:455–62.
Bhagav P, Upadhyay H, Chandran S. Brimonidine tartrate-Eudragit long-acting nanoparticles: formulation, optimization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2011;12(4):1087–101.
Khan S, Ali A, Singhavi D, Yeole P. Controlled ocular delivery of acyclovir through rate controlling ocular insert of Eudragit: a technical note. AAPS PharmSciTech. 2008;9(1):169–76.
Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–55.
Safwat SM, Al-Kassas RS. Evaluation of gentamicin-Eudragit microspheres as ophthalmic delivery systems in inflamed rabbit’s eyes. STP Pharma Sci. 2002;12:357–61.
Colo GD, Zambito Y, Burgalassi S, Serafini A, Saettone MF. Effect of chitosan on in vitro release and ocular delivery of ofloxacin from erodible inserts based on poly(ethylene oxide). Int J Pharm. 2002;248:115–22.
Harmia T, Speiser P, Kreuter J. Nanoparticles as drug carriers in ophthalmology. Pharm Acta Helv. 1986;62:322–31.
Pham-Huy C, Stathoulopoulou F, Sandouk P, Scherrmann J, Palombo S, Girre C. Rapid determination of valacyclovir and acyclovir in human biological fluids by high-performance liquid chromatography using isocratic elution. J Chromatogr B. 1999;732:47–53.
Trapani A, Laqintana V, Denora N, Lopedota A, Cutrignelli A. Eudragit RS 100 microparticles containing 2-hydroxypropyl-β-cyclodextrin and glutathione: physicochemical characterization, drug release and transport studies. Eur J Pharm Sci. 2007;30:64–74.
Miyazaki S, Suzuki S, Kawasaki N, Endo K, Takahashi A. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int J Pharm. 2001;229:29–36.
Dillen K, Vandervoort J, Mooter GV, Ludwig A. Evaluation of ciprofloxacin-loaded Eudragit® RS100 or RL100/PLGA nanoparticles. Int J Pharm. 2006;314:72–82.
Gibaud S, Al Awwadi NJ, Ducki C, Astier A. Poly(-caprolactone) and Eudragit microparticles containing fludrocortisones acetate. Int J Pharm. 2004;269:491–508.
Huang J, Wigent RJ, Bentzley CM, Schwartz JB. Nifedipine solid dispersion in microparticles of ammonio methacrylate copolymer and ethylcellulose binary blend for controlled drug delivery effect of drug loading on release kinetics. Int J Pharm. 2006;319:44–54.
Li X, Nie S, Kong J, Li N, Ju C, Pan W. A controlled-release ocular delivery system for ibuprofen based on nanostructured lipid carriers. Int J Pharm. 2008;363:177–82.
Gavini E, Chetoni P, Cossu M, Alvarez GM, Saettone FM. PLGA microspheres for the ocular delivery of a peptide drug, vancomycin using emulsification/spray-drying as the preparation method: in vitro/in vivo studies. Eur J Pharm Biopharm. 2004;57:207–12.
Schulman J, Peyman GA, Fiscella R, Greenberg D, Horton MB, Miranda PD. Intraocular acyclovir levels after subconjunctival and topical administration. Br J Ophthalmol. 1986;70:138–40.
Shen Y, Tu J. Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J. 2007;9(3):E371–7.
Bhatta RS, Chandasana H, Chhonker YS, Rathi C, Kumar D, Mitra K, et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int J Pharm. 2012;432:105–12.
Hombreiro-Perez M, Siepmann J, Zinutti C, Lamprecht A, Ubrich N, Hoffman M, et al. Non-degradable microparticles containing a hydrophilic and/or a lipophilic drug: preparation, characterization and drug release modeling. J Control Release. 2003;88:413–28.
Kristmundsdottir T, Gudmundsson OS, Ingvarsdottir K. Release of diltiazem from Eudragit microparticles prepared by spry-drying. Int J Pharm. 1996;137:159–65.
Nakano M, Lockhart CM, Kelly EJ, Rettie AE. Ocular cytochrome P450s and transporters: roles in disease and endobiotic and xenobiotic disposition. Drug Metab Rev. 2014. doi:10.3109/03602532.2014.921190.
Anand BS, Hill JM, Dey S, Maruyama K, Bhattacharjee PS. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, val-val-acyclovir, against HSV-1 epithelial and stromal keratitis in the rabbit eye model. Invest Ophthalmol Vis Sci. 2003;44(6):2529–34.
Acknowledgments
We are thankful to Dr. Ramesh K, Director, Karnataka College of Pharmacy, Bengaluru, Karnataka, India and Mr. Lokesh Prasad, DTL, Bengaluru Karnataka, India for their valuable contribution to make this research work possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Kapanigowda, U.G., Nagaraja, S.H., Ramaiah, B. et al. Enhanced Trans-Corneal Permeability of Valacyclovir by Polymethacrylic Acid Copolymers Based Ocular Microspheres: In Vivo Evaluation of Estimated Pharmacokinetic/Pharmacodynamic Indices and Simulation of Aqueous Humor Drug Concentration-Time Profile. J Pharm Innov 11, 82–91 (2016). https://doi.org/10.1007/s12247-015-9239-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-015-9239-0