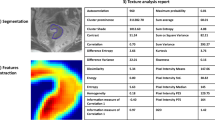
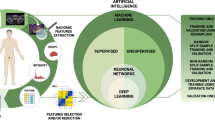
Abstract
The diagnosis of breast cancer currently relies on radiological and clinical evaluation, confirmed by histopathological examination. However, such approach has some limitations as the suboptimal sensitivity, the long turnaround time for recall tests, the invasiveness of the procedure and the risk that some features of target lesions may remain undetected, making re-biopsy a necessity. Recent technological advances in the field of artificial intelligence hold promise in addressing such medical challenges not only in cancer diagnosis, but also in treatment assessment, and monitoring of disease progression. In the perspective of a truly personalised medicine, based on the early diagnosis and individually tailored treatments, two new technologies, namely radiomics and liquid biopsy, are rising as means to obtain information from diagnosis to molecular profiling and response assessment, without the need of a biopsied tissue sample. Radiomics works through the extraction of quantitative peculiar features of cancer from radiological data, while liquid biopsy gets the whole of the malignancy’s biology from something as easy as a blood sample. Both techniques hopefully will identify diagnostic and prognostic information of breast cancer potentially reducing the need for invasive (and often difficult to perform) biopsies and favouring an approach that is as personalised as possible for each patient. Nevertheless, such techniques will not substitute tissue biopsy in the near future, and even in further times they will require the aid of other parameters to be correctly interpreted and acted upon.



Similar content being viewed by others
References
Tan W, Yang M, Yang H, Zhou F, Shen W. Predicting the response to neoadjuvant therapy for early-stage breast cancer: tumor-, blood-, and imaging-related biomarkers. Cancer Manag Res. 2018;10:4333–477. https://doi.org/10.2147/CMAR.S174435.
American Cancer Society. Breast cancer survival rates by stage. 2019. https://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-survival-by-stage. 2019.
Tirada N, Aujero M, Khorjekar G, Richards S, Chopra J, Dromi S, et al. Breast cancer tissue markers, genomic profiling, and other prognostic factors: a primer for radiologists. Radiographics. 2018;38(7):1902–20. https://doi.org/10.1148/rg.2018180047.
NCCN.org. Breast Cancer Screening and Diagnosis Version 3.2018. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2018.
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. https://doi.org/10.3121/cmr.2009.825.
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. https://doi.org/10.1056/NEJMoa022152.
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(3):310–20. https://doi.org/10.6004/jnccn.2018.0012.
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. https://doi.org/10.1158/1055-9965.EPI-15-0578.
Tagliafico AS, Piana M, Schenone D, Lai R, Massone AM, Houssami N. Overview of radiomics in breast cancer diagnosis and prognostication. Breast. 2019;49:74–80. https://doi.org/10.1016/j.breast.2019.10.018.
De Luca F, Rotunno G, Salvianti F, Galardi F, Pestrin M, Gabellini S, et al. Mutational analysis of single circulating tumor cells by next generation sequencing in metastatic breast cancer. Oncotarget. 2016;7(18):26107–19. https://doi.org/10.18632/oncotarget.8431.
Codari M, Schiaffino S, Sardanelli F, Trimboli RM. Artificial intelligence for breast MRI in 2008–2018: a systematic mapping review. AJR Am J Roentgenol. 2019;212(2):280–92. https://doi.org/10.2214/AJR.18.20389.
Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. https://doi.org/10.1186/s41747-018-0061-6.
Becker AS, Mueller M, Stoffel E, Marcon M, Ghafoor S, Boss A. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91(1083):20170576. https://doi.org/10.1259/bjr.20170576.
Becker AS, Marcon M, Ghafoor S, Wurnig MC, Frauenfelder T, Boss A. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52(7):434–40. https://doi.org/10.1097/RLI.0000000000000358.
Nance JW Jr, Meenan C, Nagy PG. The future of the radiology information system. AJR Am J Roentgenol. 2013;200(5):1064–70. https://doi.org/10.2214/AJR.12.10326.
Rotili A, Trimboli RM, Penco S, Pesapane F, Tantrige P, Cassano E, et al. Double reading of diffusion-weighted magnetic resonance imaging for breast cancer detection. Breast Cancer Res Treat. 2020. https://doi.org/10.1007/s10549-019-05519-y.
Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep Learning-Based Multi-omics integration robustly predicts survival in liver cancer. Clin Cancer Res. 2018;24(6):1248–59. https://doi.org/10.1158/1078-0432.CCR-17-0853.
Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS, et al. Predicting treatment response to intra-arterial therapies for hepatocellular carcinoma with the use of supervised machine learning-an artificial intelligence concept. J Vasc Interv Radiol. 2018. https://doi.org/10.1016/j.jvir.2018.01.769.
El-Sayed ME, Rakha EA, Reed J, Lee AH, Evans AJ, Ellis IO. Predictive value of needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Histopathology. 2008;53(6):650–7. https://doi.org/10.1111/j.1365-2559.2008.03158.x.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–62. https://doi.org/10.1038/nrclinonc.2017.141.
Yip SSF, Parmar C, Kim J, Huynh E, Mak RH, Aerts H. Impact of experimental design on PET radiomics in predicting somatic mutation status. Eur J Radiol. 2017;97:8–15. https://doi.org/10.1016/j.ejrad.2017.10.009.
Parekh VS, Jacobs MA. Integrated radiomic framework for breast cancer and tumor biology using advanced machine learning and multiparametric MRI. NPJ Breast Cancer. 2017;3:43. https://doi.org/10.1038/s41523-017-0045-3.
Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3(6):573–89. https://doi.org/10.1007/s13244-012-0196-6.
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. https://doi.org/10.1038/ncomms5006.
Rahmim A, Salimpour Y, Jain S, Blinder SA, Klyuzhin IS, Smith GS, et al. Application of texture analysis to DAT SPECT imaging: relationship to clinical assessments. Neuroimage Clin. 2016;12:e1–e9. https://doi.org/10.1016/j.nicl.2016.02.012.
Pesapane F, Patella F, Fumarola EM, Panella S, Ierardi AM, Pompili GG, et al. Intravoxel incoherent motion (IVIM) diffusion weighted imaging (DWI) in the periferic prostate cancer detection and stratification. Med Oncol. 2017;34(3):35. https://doi.org/10.1007/s12032-017-0892-7.
Patella F, Franceschelli G, Petrillo M, Sansone M, Fusco R, Pesapane F, et al. A multiparametric analysis combining DCE-MRI- and IVIM -derived parameters to improve differentiation of parotid tumors: a pilot study. Future Oncol. 2018. https://doi.org/10.2217/fon-2017-0655.
King AD, Chow KK, Yu KH, Mo FK, Yeung DK, Yuan J, et al. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013;266(2):531–8. https://doi.org/10.1148/radiol.12120167.
Peng SL, Chen CF, Liu HL, Lui CC, Huang YJ, Lee TH, et al. Analysis of parametric histogram from dynamic contrast-enhanced MRI: application in evaluating brain tumor response to radiotherapy. NMR Biomed. 2013;26(4):443–50. https://doi.org/10.1002/nbm.2882.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. https://doi.org/10.1056/NEJMoa041588.
Pizzini FB, Pesapane F, Niessen W, Geerts-Ossevoort L, Broeckx N. ESMRMB round table report on "Can Europe Lead in Machine Learning of MRI-Data?". MAGMA. 2020. https://doi.org/10.1007/s10334-019-00821-8.
Pinker K, Chin J, Melsaether AN, Morris EA, Moy L. Precision medicine and radiogenomics in breast cancer: new approaches toward diagnosis and treatment. Radiology. 2018;287(3):732–47. https://doi.org/10.1148/radiol.2018172171.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. https://doi.org/10.1126/scitranslmed.3007094.
Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501(7467):338–45. https://doi.org/10.1038/nature12625.
Xiong Q, Zhou X, Liu Z, Lei C, Yang C, Yang M, et al. Multiparametric MRI-based radiomics analysis for prediction of breast cancers insensitive to neoadjuvant chemotherapy. Clin Transl Oncol. 2019. https://doi.org/10.1007/s12094-019-02109-8.
Braman N, Prasanna P, Whitney J, Singh S, Beig N, Etesami M, et al. Association of peritumoral radiomics with tumor biology and pathologic response to preoperative targeted therapy for HER2 (ERBB2)-positive breast cancer. JAMA Netw Open. 2019;2(4):e192561. https://doi.org/10.1001/jamanetworkopen.2019.2561.
Guo Y, Hu Y, Qiao M, Wang Y, Yu J, Li J, et al. Radiomics analysis on ultrasound for prediction of biologic behavior in breast invasive ductal carcinoma. Clin Breast Cancer. 2018;18(3):e335–e34444. https://doi.org/10.1016/j.clbc.2017.08.002.
Tyagi NK, Dhesy-Thind S. Clinical practice guidelines in breast cancer. Curr Oncol. 2018;25(Suppl 1):S151–S160160. https://doi.org/10.3747/co.25.3729.
Penco S, Rizzo S, Bozzini AC, Latronico A, Menna S, Cassano E, et al. Stereotactic vacuum-assisted breast biopsy is not a therapeutic procedure even when all mammographically found calcifications are removed: analysis of 4,086 procedures. AJR Am J Roentgenol. 2010;195(5):1255–60. https://doi.org/10.2214/AJR.10.4208.
Abbate F, Bacigalupo L, Latronico A, Trentin C, Penco S, Menna S, et al. Ultrasound-guided vacuum assisted breast biopsy in the assessment of C3 breast lesions by ultrasound-guided fine needle aspiration cytology: results and costs in comparison with surgery. Breast. 2009;18(2):73–7. https://doi.org/10.1016/j.breast.2009.01.001.
Raghu M, Hooley R. Breast ultrasound for the interventionalist. Tech Vasc Interv Radiol. 2014;17(1):16–22. https://doi.org/10.1053/j.tvir.2013.12.004.
Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002;40(3):483–500. https://doi.org/10.1016/s0033-8389(01)00011-2.
Mahoney MC, Newell MS. Breast intervention: how I do it. Radiology. 2013;268(1):12–24. https://doi.org/10.1148/radiol.13120985.
Ames V, Britton PD. Stereotactically guided breast biopsy: a review. Insights Imaging. 2011;2(2):171–6. https://doi.org/10.1007/s13244-010-0064-1.
Imschweiler T, Haueisen H, Kampmann G, Rageth L, Seifert B, Rageth C, et al. MRI-guided vacuum-assisted breast biopsy: comparison with stereotactically guided and ultrasound-guided techniques. Eur Radiol. 2014;24(1):128–35. https://doi.org/10.1007/s00330-013-2989-5.
Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E, et al. Breast MRI: EUSOBI recommendations for women's information. Eur Radiol. 2015;25(12):3669–788. https://doi.org/10.1007/s00330-015-3807-z.
Le Bihan D. Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology. 2008;249(3):748–52. https://doi.org/10.1148/radiol.2493081301.
Iima M, Le Bihan D, Okumura R, Okada T, Fujimoto K, Kanao S, et al. Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology. 2011;260(2):364–72. https://doi.org/10.1148/radiol.11101892.
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. https://doi.org/10.1148/radiology.168.2.3393671.
Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–25.
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–7. https://doi.org/10.1148/radiology.161.2.3763909.
Dey D, Commandeur F. Radiomics to identify high-risk atherosclerotic plaque from computed tomography: the power of quantification. Circ Cardiovasc Imaging. 2017. https://doi.org/10.1161/CIRCIMAGING.117.007254.
Kolossvary M, Kellermayer M, Merkely B, Maurovich-Horvat P. Cardiac computed tomography radiomics: a comprehensive review on radiomic techniques. J Thorac Imaging. 2018;33(1):26–34. https://doi.org/10.1097/RTI.0000000000000268.
Barnett GC, Coles CE, Elliott RM, Baynes C, Luccarini C, Conroy D, et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: a prospective analysis study. Lancet Oncol. 2012;13(1):65–77. https://doi.org/10.1016/S1470-2045(11)70302-3.
Yard BD, Adams DJ, Chie EK, Tamayo P, Battaglia JS, Gopal P, et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat Commun. 2016;7:11428. https://doi.org/10.1038/ncomms11428.
Radiogenomics Consortium. RGC. 2009. https://epi.grants.cancer.gov/radiogenomics/.
Kuo MD, Jamshidi N. Behind the numbers: decoding molecular phenotypes with radiogenomics—guiding principles and technical considerations. Radiology. 2014;270(2):320–5. https://doi.org/10.1148/radiol.13132195.
Rutman AM, Kuo MD. Radiogenomics: creating a link between molecular diagnostics and diagnostic imaging. Eur J Radiol. 2009;70(2):232–41. https://doi.org/10.1016/j.ejrad.2009.01.050.
Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–22. https://doi.org/10.1158/1078-0432.CCR-10-2692.
Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754–65. https://doi.org/10.1056/NEJMra1706174.
Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. https://doi.org/10.1056/NEJMoa1113205.
Emaminejad N, Qian W, Guan Y, Tan M, Qiu Y, Liu H, et al. Fusion of quantitative image and genomic biomarkers to improve prognosis assessment of early stage lung cancer patients. IEEE Trans Biomed Eng. 2016;63(5):1034–43. https://doi.org/10.1109/TBME.2015.2477688.
Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–91. https://doi.org/10.1016/S1470-2045(18)30413-3.
Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, et al. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25(10):2840–50. https://doi.org/10.1007/s00330-015-3701-8.
Fehr D, Veeraraghavan H, Wibmer A, Gondo T, Matsumoto K, Vargas HA, et al. Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc Natl Acad Sci USA. 2015;112(46):E6265–E62736273. https://doi.org/10.1073/pnas.1505935112.
Coroller TP, Bi WL, Huynh E, Abedalthagafi M, Aizer AA, Greenwald NF, et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS ONE. 2017;12(11):e0187908. https://doi.org/10.1371/journal.pone.0187908.
Hanania AN, Bantis LE, Feng Z, Wang H, Tamm EP, Katz MH, et al. Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget. 2016;7(52):85776–84. https://doi.org/10.18632/oncotarget.11769.
Parikh J, Selmi M, Charles-Edwards G, Glendenning J, Ganeshan B, Verma H, et al. Changes in primary breast cancer heterogeneity may augment midtreatment MR imaging assessment of response to neoadjuvant chemotherapy. Radiology. 2014;272(1):100–12. https://doi.org/10.1148/radiol.14130569.
Pickles MD, Lowry M, Gibbs P. Pretreatment prognostic value of dynamic contrast-enhanced magnetic resonance imaging vascular, texture, shape, and size parameters compared with traditional survival indicators obtained from locally advanced breast cancer patients. Invest Radiol. 2016;51(3):177–85. https://doi.org/10.1097/RLI.0000000000000222.
Kim JH, Ko ES, Lim Y, Lee KS, Han BK, Ko EY, et al. Breast cancer heterogeneity: MR imaging texture analysis and survival outcomes. Radiology. 2017;282(3):665–75. https://doi.org/10.1148/radiol.2016160261.
Li H, Zhu Y, Burnside ES, Huang E, Drukker K, Hoadley KA, et al. Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer. 2016. https://doi.org/10.1038/npjbcancer.2016.12.
Holli K, Laaperi AL, Harrison L, Luukkaala T, Toivonen T, Ryymin P, et al. Characterization of breast cancer types by texture analysis of magnetic resonance images. Acad Radiol. 2010;17(2):135–41. https://doi.org/10.1016/j.acra.2009.08.012.
Wang J, Kato F, Oyama-Manabe N, Li R, Cui Y, Tha KK, et al. Identifying triple-negative breast cancer using background parenchymal enhancement heterogeneity on dynamic contrast-enhanced MRI: a pilot radiomics study. PLoS ONE. 2015;10(11):e0143308. https://doi.org/10.1371/journal.pone.0143308.
Fan M, Li H, Wang S, Zheng B, Zhang J, Li L. Radiomic analysis reveals DCE-MRI features for prediction of molecular subtypes of breast cancer. PLoS ONE. 2017;12(2):e0171683. https://doi.org/10.1371/journal.pone.0171683.
Zhu Y, Li H, Guo W, Drukker K, Lan L, Giger ML, et al. Deciphering genomic underpinnings of quantitative MRI-based radiomic phenotypes of invasive breast carcinoma. Sci Rep. 2015;5:17787. https://doi.org/10.1038/srep17787.
James D, Clymer BD, Schmalbrock P. Texture detection of simulated microcalcification susceptibility effects in magnetic resonance imaging of breasts. J Magn Reson Imaging. 2001;13(6):876–81.
Dong Y, Feng Q, Yang W, Lu Z, Deng C, Zhang L, et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2-weighted fat-suppression and diffusion-weighted MRI. Eur Radiol. 2018;28(2):582–91. https://doi.org/10.1007/s00330-017-5005-7.
Bickelhaupt S, Paech D, Kickingereder P, Steudle F, Lederer W, Daniel H, et al. Prediction of malignancy by a radiomic signature from contrast agent-free diffusion MRI in suspicious breast lesions found on screening mammography. J Magn Reson Imaging. 2017;46(2):604–16. https://doi.org/10.1002/jmri.25606.
Li H, Zhu Y, Burnside ES, Drukker K, Hoadley KA, Fan C, et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of mammaprint, oncotype DX, and PAM50 gene assays. Radiology. 2016;281(2):382–91. https://doi.org/10.1148/radiol.2016152110.
Obeid JP, Stoyanova R, Kwon D, Patel M, Padgett K, Slingerland J, et al. Multiparametric evaluation of preoperative MRI in early stage breast cancer: prognostic impact of peri-tumoral fat. Clin Transl Oncol. 2017;19(2):211–8. https://doi.org/10.1007/s12094-016-1526-9.
Fan M, Wu G, Cheng H, Zhang J, Shao G, Li L. Radiomic analysis of DCE-MRI for prediction of response to neoadjuvant chemotherapy in breast cancer patients. Eur J Radiol. 2017;94:140–7. https://doi.org/10.1016/j.ejrad.2017.06.019.
Zhou J, Zhang Y, Chang KT, Lee KE, Wang O, Li J, et al. Diagnosis of benign and malignant breast lesions on DCE-MRI by using radiomics and deep learning with consideration of peritumor tissue. J Magn Reson Imaging. 2019. https://doi.org/10.1002/jmri.26981.
Liu J, Sun D, Chen L, Fang Z, Song W, Guo D, et al. Radiomics analysis of dynamic contrast-enhanced magnetic resonance imaging for the prediction of sentinel lymph node metastasis in breast cancer. Front Oncol. 2019;9:980. https://doi.org/10.3389/fonc.2019.00980.
Park H, Lim Y, Ko ES, Cho HH, Lee JE, Han BK, et al. Radiomics signature on magnetic resonance imaging: association with disease-free survival in patients with invasive breast cancer. Clin Cancer Res. 2018;24(19):4705–14. https://doi.org/10.1158/1078-0432.CCR-17-3783.
Antunovic L, Gallivanone F, Sollini M, Sagona A, Invento A, Manfrinato G, et al. [(18)F]FDG PET/CT features for the molecular characterization of primary breast tumors. Eur J Nucl Med Mol Imaging. 2017;44(12):1945–54. https://doi.org/10.1007/s00259-017-3770-9.
Ou X, Zhang J, Wang J, Pang F, Wang Y, Wei X, et al. Radiomics based on (18) F-FDG PET/CT could differentiate breast carcinoma from breast lymphoma using machine-learning approach: A preliminary study. Cancer Med. 2019. https://doi.org/10.1002/cam4.2711.
Sakai A, Onishi Y, Matsui M, Adachi H, Teramoto A, Saito K, et al. A method for the automated classification of benign and malignant masses on digital breast tomosynthesis images using machine learning and radiomic features. Radiol Phys Technol. 2019. https://doi.org/10.1007/s12194-019-00543-5.
Teruel JR, Heldahl MG, Goa PE, Pickles M, Lundgren S, Bathen TF, et al. Dynamic contrast-enhanced MRI texture analysis for pretreatment prediction of clinical and pathological response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. NMR Biomed. 2014;27(8):887–96. https://doi.org/10.1002/nbm.3132.
Antunovic L, De Sanctis R, Cozzi L, Kirienko M, Sagona A, Torrisi R, et al. PET/CT radiomics in breast cancer: promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2019;46(7):1468–77. https://doi.org/10.1007/s00259-019-04313-8.
Cain EH, Saha A, Harowicz MR, Marks JR, Marcom PK, Mazurowski MA. Multivariate machine learning models for prediction of pathologic response to neoadjuvant therapy in breast cancer using MRI features: a study using an independent validation set. Breast Cancer Res Treat. 2018. https://doi.org/10.1007/s10549-018-4990-9.
Hilal T, Covington M, Kosiorek HE, Zwart C, Ocal IT, Pockaj BA, et al. Breast MRI phenotype and background parenchymal enhancement may predict tumor response to neoadjuvant endocrine therapy. Breast J. 2018;24(6):1010–4. https://doi.org/10.1111/tbj.13101.
Drukker K, Li H, Antropova N, Edwards A, Papaioannou J, Giger ML. Most-enhancing tumor volume by MRI radiomics predicts recurrence-free survival "early on" in neoadjuvant treatment of breast cancer. Cancer Imaging. 2018;18(1):12. https://doi.org/10.1186/s40644-018-0145-9.
Pesapane F, Volonte C, Codari M, Sardanelli F. Artificial intelligence as a medical device in radiology: ethical and regulatory issues in Europe and the United States. Insights Imaging. 2018. https://doi.org/10.1007/s13244-018-0645-y.
Ranschaert ER, Sergey M, Algra PR. Artificial intelligence in medical imaging. Berlin: Springer; 2019.
Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–64. https://doi.org/10.1038/nature12627.
Ashworth T. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869;14:146–9.
Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the Cell Search system. Mol Oncol. 2016;10(3):395–407. https://doi.org/10.1016/j.molonc.2015.12.002.
Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18(20):5701–10. https://doi.org/10.1158/1078-0432.CCR-12-1587.
Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–14. https://doi.org/10.1016/S1470-2045(14)70069-5.
Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a Meta-analysis. J Natl Cancer Inst. 2018;110(6):560–7. https://doi.org/10.1093/jnci/djy018.
Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4(6):650–61. https://doi.org/10.1158/2159-8290.CD-13-1014.
Sun W, Yuan X, Tian Y, Wu H, Xu H, Hu G, et al. Non-invasive approaches to monitor EGFR-TKI treatment in non-small-cell lung cancer. J Hematol Oncol. 2015;8:95. https://doi.org/10.1186/s13045-015-0193-6.
Canzoniero JV, Park BH. Use of cell free DNA in breast oncology. Biochim Biophys Acta. 2016;1865(2):266–74. https://doi.org/10.1016/j.bbcan.2016.03.006.
Catarino R, Ferreira MM, Rodrigues H, Coelho A, Nogal A, Sousa A, et al. Quantification of free circulating tumor DNA as a diagnostic marker for breast cancer. DNA Cell Biol. 2008;27(8):415–21. https://doi.org/10.1089/dna.2008.0744.
Shaw JA, Page K, Blighe K, Hava N, Guttery D, Ward B, et al. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res. 2012;22(2):220–31. https://doi.org/10.1101/gr.123497.111.
De Giorgi V, Pinzani P, Salvianti F, Grazzini M, Orlando C, Lotti T, et al. Circulating benign nevus cells detected by ISET technique: warning for melanoma molecular diagnosis. Arch Dermatol. 2010;146(10):1120–4. https://doi.org/10.1001/archdermatol.2010.264.
Shan M, Yin H, Li J, Li X, Wang D, Su Y, et al. Detection of aberrant methylation of a six-gene panel in serum DNA for diagnosis of breast cancer. Oncotarget. 2016;7(14):18485–944. https://doi.org/10.18632/oncotarget.7608.
Oshiro C, Kagara N, Naoi Y, Shimoda M, Shimomura A, Maruyama N, et al. PIK3CA mutations in serum DNA are predictive of recurrence in primary breast cancer patients. Breast Cancer Res Treat. 2015;150(2):299–307. https://doi.org/10.1007/s10549-015-3322-6.
Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, Inao T, Sueta A, Fujiwara S, et al. Prognostic role of PIK3CA mutations of cell-free DNA in early-stage triple negative breast cancer. Cancer Sci. 2015;106(11):1582–9. https://doi.org/10.1111/cas.12813.
Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7(302):302ra133. https://doi.org/10.1126/scitranslmed.aab0021.
Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MH, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015;7(8):1034–47. https://doi.org/10.15252/emmm.201404913.
Guttery DS, Page K, Hills A, Woodley L, Marchese SD, Rghebi B, et al. Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer. Clin Chem. 2015;61(7):974–82. https://doi.org/10.1373/clinchem.2015.238717.
Chu D, Paoletti C, Gersch C, VanDenBerg DA, Zabransky DJ, Cochran RL, et al. ESR1 Mutations in circulating plasma tumor DNA from metastatic breast cancer patients. Clin Cancer Res. 2016;22(4):993–9. https://doi.org/10.1158/1078-0432.CCR-15-0943.
Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014. https://doi.org/10.1093/jnci/dju066.
Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32(31):3483–9. https://doi.org/10.1200/JCO.2014.56.2561.
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA. 2004;101(25):9393–8. https://doi.org/10.1073/pnas.0402993101.
Georgoulias V, Bozionelou V, Agelaki S, Perraki M, Apostolaki S, Kallergi G, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23(7):1744–50. https://doi.org/10.1093/annonc/mds020.
Board RE, Thelwell NJ, Ravetto PF, Little S, Ranson M, Dive C, et al. Multiplexed assays for detection of mutations in PIK3CA. Clin Chem. 2008;54(4):757–60. https://doi.org/10.1373/clinchem.2007.098376.
Board RE, Wardley AM, Dixon JM, Armstrong AC, Howell S, Renshaw L, et al. Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat. 2010;120(2):461–7. https://doi.org/10.1007/s10549-010-0747-9.
Madic J, Piperno-Neumann S, Servois V, Rampanou A, Milder M, Trouiller B, et al. Pyrophosphorolysis-activated polymerization detects circulating tumor DNA in metastatic uveal melanoma. Clin Cancer Res. 2012;18(14):3934–41. https://doi.org/10.1158/1078-0432.CCR-12-0309.
Higgins MJ, Jelovac D, Barnathan E, Blair B, Slater S, Powers P, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18(12):3462–9. https://doi.org/10.1158/1078-0432.CCR-11-2696.
Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3(7):551–9. https://doi.org/10.1038/nmeth898.
Beaver JA, Jelovac D, Balukrishna S, Cochran R, Croessmann S, Zabransky DJ, et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res. 2014;20(10):2643–50. https://doi.org/10.1158/1078-0432.CCR-13-2933.
Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–10. https://doi.org/10.1021/ac202028g.
Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4(136):136. https://doi.org/10.1126/scitranslmed.3003726.
Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108(23):9530–5. https://doi.org/10.1073/pnas.1105422108.
Fox EJ, Reid-Bayliss KS, Emond MJ, Loeb LA. Accuracy of next generation sequencing platforms. Next Gener Seq Appl. 2014. https://doi.org/10.4172/jngsa.1000106.
Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA. Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci USA. 2012;109(36):14508–13. https://doi.org/10.1073/pnas.1208715109.
Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2(20):20. https://doi.org/10.1126/scitranslmed.3000702.
Leary RJ, Sausen M, Kinde I, Papadopoulos N, Carpten JD, Craig D, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4(162):162. https://doi.org/10.1126/scitranslmed.3004742.
Acknowledgements
This manuscript was concepted and designed by the Artificial Intelligence for Oncology (AIFO) working group.
Funding
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds.
Author information
Authors and Affiliations
Contributions
Conception and design: Dr. FP and Dr. MS (AIFO WG); Administrative support: Dr. EC; Provision of study materials or patients: All authors; Collection and assembly of data: All authors; Data analysis and interpretation: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
All author declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pesapane, F., Suter, M.B., Rotili, A. et al. Will traditional biopsy be substituted by radiomics and liquid biopsy for breast cancer diagnosis and characterisation?. Med Oncol 37, 29 (2020). https://doi.org/10.1007/s12032-020-01353-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01353-1