Abstract
The purpose of this study was to evaluate expression and prognostic impact of Nanog, Oct4, Sox2, proliferation cell nuclear antigen (PCNA), Ki67 and E-cadherin in patients with gastric cancer (GC) by immunohistochemistry. A total of 69 patients were recruited who underwent gastrectomy between 2008 and 2009. We found that expression levels of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin were 26.1, 53.6, 49.3, 52.2, 60.9 and 60.9 %, respectively. Co-expression of more than any two proteins (defined as high-risk group) was detected in 43 of 69 (62.3 %) patients with GC. Only positive expression of Oct4 had relationship with lymphatic invasion (p = 0.013), and positive expression of Ki67 was correlated with T classification (p = 0.011). Furthermore, positive expression of Oct4 (p = 0.043), PCNA (p = 0.035) and Ki67 (p = 0.023) was significantly associated with poor 3-year disease-free survival (DFS). The same result was detected in patients with E-cadherin reduced expression (p = 0.022). But only PCNA positive expression predicted poor overall survival (p = 0.042) in univariate analysis. In addition, 3-year DFS was 20 % in high-risk group and 71 % in low-risk group. The same tendency was found between OS and co-expression of proteins. There was a remarkable difference between DFS or OS and co-expression of more than two proteins (p = 0.000). Multivariate analysis showed that E-cadherin and co-expression were independent prognostic factors of 3-year diseases-free survival. But only co-expression of more than two markers dramatically affected the survival of GC patients. These findings provide evidence that combined evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin may be a more powerful prognostic factor to predict relapse and distant metastasis for patients with GC.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer deaths worldwide, although the incidence and mortality rates have recently been declining [1]. Complete resection of cancer is the only way to cure GC. Chemotherapy and radiotherapy both are important adjuvant treatment and have been shown to increase survival after resection. But unfortunately, a high rate of locoregional and distant recurrences has been reported. The site of recurrence is local in 19–42 % of cases, peritoneal in 21–72 % and distant in 18–49 % [2]. Although clinical stage, histological differentiation and the depth of invasion provide much helpful information to estimate patients’ survival time, there is still a lack of biological molecular markers to precisely detect or predict relapse and metastasis.
The emergence of cancer stem cells (CSCs) theory throws light on the mechanism of recurrence after curative resection and adjuvant treatment. CSCs, which are characterized by the highest potential for self-renewal and proliferation, have been discovered in many solid tumors [3]. Various molecules are being investigated as putative markers of CSCs in malignancies [4]. Nanog, Oct4 and Sox2 are significant transcription factors in the maintenance of self-renewal and pluripotency of stem cells [5]. They express more frequently in poorly differentiated tumors than in well-differentiated tumors and contribute to aggressiveness and poor prognosis of cancer [6, 7]. Although CSCs may be associated with recurrence and metastasis, epithelial-to-mesenchymal transition (EMT) may give CSCs the invasive and metastatic abilities necessary for successful metastasis [8]. EMT is another central element of tumor migration. And metastasis is also associated with reduced expression of E-cadherin in tumor [8, 9]. Another remarkable characteristic of cancer cells is malignant proliferation. Ki67 and proliferation cell nuclear antigen (PCNA) are usually recognized as useful markers for proliferation of tumor cells. Ki67 is synthesized at the beginning of cell proliferation and expresses in all phases of the cell cycle except G0 phase [10]. PCNA is an intro-nuclear protein that is closely linked to the cell cycle [11]. Many studies have shown that Ki67 is a valuable prognostic and predictive marker [12]. But there are conflicting results regarding the correlation between PCNA expression in tumor tissues and prognosis [13].
Taken together, growing data support that CSCs, EMT and proliferation have important roles in relapse and metastasis. However, most of data are based on different cell lines, and there is a lack of clinical implications for GC. In this study, we aim to clarify the prognostic value of CSCs, EMT and proliferation in GC patients who had undergone curative resection. The relationship between protein expression levels and clinicopathologic characteristics is also further examined.
Materials and methods
Patients and samples
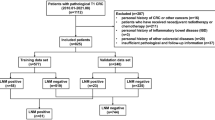
Medical records from 2008 to 2009 were retrospectively reviewed at Henan Cancer Hospital. The criteria for case inclusion were as follows: (1) Patients were diagnosed as GC without distant metastasis and had undergone complete surgery with D2 lymph node dissection; (2) all patients received more than four cycles of adjuvant chemotherapy. The regimen of chemotherapy was fluorouracil and/or cisplatin/oxaliplatin; and (3) abundant and accurate clinical details and follow-up data. The follow-up program, which consisted of physical examination and regular abdominal CT scan, was performed every 3–6 months for the first 2 years after operation and every 6–12 months thereafter. Evidence of relapse or metastasis was diagnosed by biopsy if patients permitted. A total of 69 patients were recruited in the end. TNM stages were classified according to the 7th American Joint Committee on Cancer (AJCC). The disease-free survival (DFS) was calculated between the date of surgery and the date of relapse, metastasis or the last follow-up. Death or clinical diagnosis of recurrence was recognized as end point of DFS. The overall survival (OS) was determined as period between the date of diagnosis and the date of death. The median follow-up period was 35 months (range 6–50 months). The study was approved by the Ethic Review Committee at Henan Cancer Hospital.
Immunohistochemistry
Immunohistochemical analysis was used to evaluate expression of different markers. Antibodies were obtained from different sources. The detailed information and conditions for all antibodies were listed in Table 1. All markers were detected using published method [14]. Sections (4 μm) from each sample were deparaffinized in xylene, dehydrated in a graded series of alcohol and rehydrated in Tris-buffered saline (TBS) (pH 7.5). Then, the sections were pre-treated by a microwave, treated with 3 % hydrogen peroxide and incubated with primary antibodies overnight at 4 °C. After washing with TBS, the slides were incubated with the second antibody for 45 min then washed again and treated with 3, 3′-diaminobenzidine tetrahydrochloride (DAB) until staining was optimal as determined by light microscopic examination, then finally counter-stained with hematoxylin. Negative controls for these proteins were made by the omission of the primary antibody during the procedure of immunohistochemical staining. Two pathologists, who were blinded to the clinical characteristics of patients, independently reviewed the slides and were in agreement with the extent of immunohistochemical staining.
Nanog, Oct4 and Sox2 mainly expressed in the nucleus of GC cells and also slightly expressed in the cytoplasm. PCNA and Ki67 were located in the nucleus. Staining for E-cadherin was observed mainly in the cell membrane. The percentage of positive staining of cells was counted in more than five fields of each cancerous tissue at high magnification (400×). For Nanog, Oct4 and Sox2, cases were scored based on the staining intensity and the percentage of cells stained (0–100 %). Staining intensity was graded from 0 to 3 (0 = no; 1 = weak; 2 = moderate; and 3 = intense). The percentage of positive cells was given scores 0–5 (0 = 0 %; 1 = 1–25 %; 2 = 26–35 %; 3 = 36–45 %; 4 = 46–65 %; and 5 = 66–100 %). The sum of intensity and percentage was the final score. A score ≥5 was considered to be indicative of positive staining of CSCs markers [15]. For E-cadherin, cases with definite membrane staining in <30 % of the tumor cells, or with a complete absence of membrane staining, were categorized as negative staining [16]. For PCNA and Ki67, more than 50 % positive staining in the nucleus was defined as positive staining [17].
Statistical analysis
All statistical data were analyzed by using SPSS 17.0 software (SPSS Inc. Chicago, IL). The Chi-square test and Fisher’s exact test (when cells had expected count <5) were used to evaluate comparisons of clinicopathologic characteristics. The Kaplan–Meier method was used to analyze OS and DFS. The log-rank test analyzed the differences between proteins expression and survival curves. Multivariate survival analysis (Cox proportional hazards regression) was performed to confirm the factors proven significant in Kaplan–Meier analysis. p values <0.05 were defined as significant.
Results
Patients’ characteristics and relationships with CSCs markers expression
The main clinical features of 69 patients and their relationships with CSCs markers (Nanog, Oct4 and Sox2) were shown in Table 2. The median age of the patients was 55 years (range 28–78 years). Fifty-one males and 18 females were included in this study. The constituent ratio of patients with stage I, stage II and stage III was 2.9 % (2/69), 31.9 % (22/69) and 65.2 % (45/69), respectively. At the end of the study, 43 patients (62.3 %) had relapsed or developed distant metastasis.
All patients were assessed for CSCs markers. As shown in Table 2, Nanog, Oct4 and Sox2 positive expression rates in GC tissues were 26.1 % (18/69), 53.6 % (37/69) and 49.3 % (34/69), respectively (Fig. 1a–c). Expression levels of Nanog, Sox2 and Oct4 did not correlate with patient gender, age, grade of differentiation, T classification and tumor stage. Significant difference was detected between N classification and Oct4 positive expression (p = 0.013).
Representative staining of Nanog (a), Oct4 (b), Sox2 (c), PCNA (d), Ki67 (e) and E-cadherin (f) by IHC. Expression of Nanog, Oct4 and Sox2 was mainly localized in the nuclei and faintly in the cytoplasm (brown, a–c). PCNA and Ki67 were located in the nucleus (d, e). Staining for E-cadherin was observed mainly in the cell membrane (f)
Expression of proliferation markers, E-cadherin and relationship with clinical characteristics
Positive expression of proliferation markers, including PCNA and Ki67, was 52.2 % (36/69) and 60.9 % (42/69), respectively (Table 2, Fig. 1d, e). There was no relationship between PCNA or Ki67 expression level and clinicopathological characteristics such as gender, age, grade, tumor stage and so on. But T classification had significant correlation with Ki67 expression (p = 0.011). In all 69 samples, 42 (60.9 %) patients were detected positive staining of E-cadherin (Fig. 1f). As shown in Table 2, gender, age, histologic grade and stage did not affect the expression of E-cadherin.
Association of clinicopathological features with co-expression of proteins
To examine the correlation between co-expression of markers and patients’ clinical characteristics, 69 patients were divided into low-risk group (which was positive for equal or less than two markers) and high-risk group (which was positive for more than two markers). In addition, E-cadherin positive expression was defined as reduced expression at this moment. As summarized in Table 2, 43 (62.3 %) cases were in high-risk group. But, there was no significant relationship between co-expression and clinical characteristics.
Correlations between markers expression and 3-year DFS or OS
The possible prognostic factors for relapse and OS were assessed by univariate and multivariate analyses. Results of the univariate analysis were summarized in Table 3 and Figs. 2, 3, 4. Based on Kaplan–Meier analysis, there was a significant difference between patients with positive expression of Oct4 and those with negative expression (3-year DFS 51 % vs. 3-year DFS 31.5 %, p = 0.043 Fig. 2b). Although 3-year DFS was better in Nanog or Sox2 negative expression than that of positive expression (Nanog: 45 vs. 24.5 %, Sox2: 47.5 vs. 34 %, Fig. 2a, c), the difference was not significant. Further analysis showed that 3-year OS of negative expression of Nanog, Oct4 and Sox2 was 61, 63, 70.5 %, respectively, and better than that of positive expression of Nanog, Oct4 and Sox2 (50, 52.5, 42.5 %, Fig. 3a–c). As the same tendency of DFS, 3-year OS was decreasing with the positive expression of CSCs markers. But the difference was of no significance. In addition, the worse 3-year DFS and OS were calculated in positive expression of PCNA (28 and 39.5 %, Figs. 2d, 3d). Compared with negative expression, the prognostic difference was significant. The same result had been detected between 3-year DFS and Ki67 expression. The 3-year DFS in Ki67 positive expression group was 30 % and 57 % in negative expression group (p = 0.023, Fig. 2e). However, significant difference was not observed between OS and Ki67 expression (p = 0.058, Fig. 3e). Furthermore, 3-year DFS of positive expression of E-cadherin was 51 % better than that of negative expression (51 vs. 21.5 %, p = 0.022, Fig. 2f). There was a trend that patients with negative expression of E-cadherin would have poor OS (58.5 %). But the difference between OS and E-cadherin expression was of no significance (p = 0.286, Fig. 3f). However, 3-year DFS of low-risk group was obviously better than that of high-risk group (71 vs 20 %, p = 0.000, Fig. 4a). Similarly, the categories were also associated with OS that 3-year survival was 88 % in low-risk group compared with 41 % in high-risk group (p = 0.000, Fig. 4b).
Kaplan–Meier survival analysis for Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin. There was no significant difference between Nanog or Sox2 expression and DFS (a, c). Patients with positive expression of Oct4 (b), PCNA (d), Ki67 (e) and reduced expression of E-cadherin (f) showed the shorter DFS. p values were calculated by log-rank test
Overall survival curves according to proteins expression in 69 patients with GC. The worse OS was calculated in positive expression of PCNA (d). There was a trend that patients with negative expression of E-cadherin (f), positive expression of other markers (a, b, c and e) would have a poor overall survival rate. But the difference was not significant
DFS and OS according to high-risk group (>2 markers positive expression) versus low-risk group (≤2 markers positive expression). E-cadherin positive expression was defined as loss of expression. Univariate analysis showed that 3-year DFS and OS of patients were worse in high-risk group than those in low-risk group (a, b)
To further confirm the independent prognostic factors for DFS and OS in all 69 patients, Cox proportional hazard model was performed as multivariate analysis (Table 3). All statistically significant prognostic factors detected at univariate analysis were included. As shown in Table 3, co-expression of more than two markers (p = 0.042, RR = 2.816) and loss of expression of E-cadherin (p = 0.013, RR = 0.401), demonstrated by multivariate analysis, were significant predictors of 3-year DFS. However, although PCNA and co-expression of proteins were significantly correlated with OS in univariate analysis, only co-expression of proteins remarkably affected OS (p = 0.002, RR = 5.084).
Discussion
Local relapse and distant metastasis threaten survival of patients with GC. Although adjuvant chemotherapy and chemoradiation reduce recurrence and metastasis, there are at least 20 % patients who developed a new carcinoma after resection [18]. The OS of GC is obviously lower than other solid tumors. Many mechanisms could be used to illustrate the procedure of recurrence such as cancer stem cell theory, EMT, activation of signaling pathway and so on. However, these mechanisms should be confirmed by clinical practice, and there is an urgent need for more sensitive markers to predict the prognosis of patients with GC.
CSCs are defined as a subpopulation that initiate tumor growth, sustain self-renewal, compose the small reservoir of drug-resistant cells and be responsible for relapse or distant metastasis after chemotherapy [19]. Increased expression of Nanog, Sox2 or Oct4 has been reported to lead to activation of growth factors, promotion of cellular proliferation and metastasis in several types of malignancies [20–24]. In the present investigation, we showed that only positive expression of Oct4 correlated with N classification. A univariate analysis revealed a significant correlation between DFS and Oct4 positive expression. But no statistical difference was found by multivariate analysis. These findings suggested that only Oct4, compared with Nanog and Sox2, might be a useful prognostic factor for relapse or distant metastasis after operation.
There are a number of articles that showed the EMT program served an important role during cancer progression. It seems to provide cancer cells with the capacity to infiltrate the surrounding tissue and ultimately metastasize to distant organs [8]. Decreased E-cadherin expression is significantly associated with histopathological features and might be a predicative factor of poor prognosis in GC patients [25]. Our analysis indicated that patients with E-cadherin negative expression had poor 3-year DFS and OS when compared to patients with E-cadherin positive expression. However, reduced expression of E-cadherin in tumor was only significantly associated with DFS in a multivariate analysis.
Ki67 has been declared as a predictor of cell proliferation, malignant potentials and recurrence of cancer. Expression level of Ki67 is also valuable for predicting the response of adjuvant chemotherapy [10]. PCNA has been reported as a powerful prognostic factor for patients with GC [26]. However, some studies imply that PCNA has no relationship with survival [27, 28]. Our results demonstrated that Ki67 expression was associated with T classification and relapse. In addition, Ki67 or PCNA positive expression predicted the worse 3-year DFS. But, neither PCNA nor Ki67 could be utilized as a valuable predictor of survival of patients.
Although univariate analysis predicted that the independent prognostic factors for DFS were increased expression of Oct4, PCNA, Ki67 and loss of E-cadherin, only reduced expression of E-cadherin was significant when analyzed by multivariate regression analysis. And each powerful marker could not individually predict OS of GC. However, the most important finding in this study was that if patients with GC had positive co-expression of more than two markers (loss of expression of E-cadherin was defined as positive expression), their DFS and OS would be inferior. The strong statistical significance was evidenced by Cox proportional regression model. To our knowledge, cancer cells relapse or metastasis is a very complex process including cell transformation, growth, angiogenesis, invasion, dissemination and survival in the circulation, and subsequent adhesion and colonization of the secondary organ or tissue [29]. Thus, the procedure of relapse is so complex that single biomarker cannot predict correctly. In other words, combined evaluation of proteins could be a more powerful prognostic factor for patients with GC after resection.
In our study, expression levels of CSCs markers, EMT markers and proliferation markers in 69 GC patients were detected. At the same time, the relationships with DFS and OS also were investigated. The results demonstrate that combined detection method may be a more valuable predictor for relapse and OS after operation. In addition, further investigation in molecular mechanism how relapse and metastasis of GC are promoted by these markers and a further analysis with a larger sample are essential to verify the results in our study.
References
Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–44.
Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–49.
Xu G, Shen J, Ou Yang X, Sasahara M, Su X. Cancer stem cells: the ‘heartbeat’ of gastric cancer. J Gastroenterol. 2013;48(7):781–97.
Shien K, Toyooka S, Ichimura K, Soh J, Furukawa M, Maki Y, et al. Prognostic impact of cancer stem cell-related markers in non-small cell lung cancer patients treated with induction chemoradiotherapy. Lung Cancer. 2012;77(1):162–7.
Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10(4):440–54.
Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40(5):499–507.
Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8(2):e56324.
Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14(1):29–43.
Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Transl Med. 2014;3:17.
He X, Chen Z, Fu T, Jin X, Yu T, Liang Y, et al. Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer. 2014;14:153.
Kanaji S, Saito H, Tsujitani S, Matsumoto S, Tatebe S, Kondo A, et al. Expression of polo-like kinase 1 (PLK1) protein predicts the survival of patients with gastric carcinoma. Oncology. 2006;70(2):126–33.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–83.
Wang SC. PCNA: a silent housekeeper or a potential therapeutic target? Trends Pharmacol Sci. 2014;35(4):178–86.
Wang L, Ma J, Liu F, Yu Q, Chu G, Perkins AC, et al. Expression of MUC1 in primary and metastatic human epithelial ovarian cancer and its therapeutic significance. Gynecol Oncol. 2007;105(3):695–702.
Matsuoka J, Yashiro M, Sakurai K, Kubo N, Tanaka H, Muguruma K, et al. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res. 2012;174(1):130–5.
Hayashi A, Yashima K, Takeda Y, Sasaki S, Kawaguchi K, Harada K, et al. Fhit, E-cadherin, p53, and activation-induced cytidine deaminase expression in endoscopically resected early stage esophageal squamous neoplasia. J Gastroenterol Hepatol. 2012;27(11):1752–8.
Mateoiu C, Pirici A, Bogdan F. Immunohistochemical nuclear staining for p53, PCNA, Ki-67 and bcl-2 in different histologic variants of basal cell carcinoma. Rom J Morphol Embryol. 2011;52(1 Suppl):315–9.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label randomised controlled trial. Lancet. 2012;379(9813):315–21.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61.
Chen Z, Xu WR, Qian H, Zhu W, Bu XF, Wang S, et al. Oct4, a novel marker for human gastric cancer. J Surg Oncol. 2009;99(7):414–9.
Lin T, Ding YQ, Li JM. Overexpression of Nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med Oncol. 2012;29(2):878–85.
Zhan YY, He JP, Chen HZ, Wang WJ, Cai JC. Orphan receptor TR3 is essential for the maintenance of stem-like properties in gastric cancer cells. Cancer Lett. 2013;329(1):37–44.
Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, Hirakawa K. Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci. 2009;100(8):1397–402.
Ji W, Jiang Z. Effect of shRNA-mediated inhibition of Nanog gene expression on the behavior of human gastric cancer cells. Oncol Lett. 2013;6(2):367–74.
Xing X, Tang YB, Yuan G, Wang Y, Wang J, Yang Y, et al. The prognostic value of E-cadherin in gastric cancer: a meta-analysis. Int J Cancer. 2013;132(11):2589–96.
Czyzewska J, Guzińska-Ustymowicz K, Pryczynicz A, Kemona A, Bandurski R. Immunohistochemical evaluation of Ki-67, PCNA and MCM2 proteins proliferation index (PI) in advanced gastric cancer. Folia Histochem Cytobiol. 2009;47(2):289–96.
Grossi F, Loprevite M, Chiaramondia M, Ceppa P, Pera C, Ratto GB, et al. Prognostic significance of K-ras, p53, bcl-2, PCNA, CD34 in radically resected non-small cell lung cancers. Eur J Cancer. 2003;39(9):1242–50.
Dworakowska D, Gózdz S, Jassem E, Badzio A, Kobierska G, Urbaniak A, et al. Prognostic relevance of proliferating cell nuclear antigen and p53 expression in non-small cell lung cancer. Lung Cancer. 2002;35(1):35–41.
Jin X, Zhu Z, Shi Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer. 2014;101(1):E1–12.
Acknowledgments
The authors would like to thank all patients for their cooperation. This study was supported by grants from the National Natural Science Foundation of China (No. 81470287) and the National Natural Science Foundation of China (No. 81370661).
Conflict of interest
The authors declared no potential conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., Deng, W., Ma, J. et al. Prognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol 32, 433 (2015). https://doi.org/10.1007/s12032-014-0433-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0433-6