Abstract
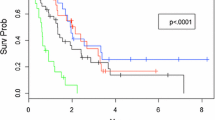
HER2-positive breast cancer is a known risk factor for CNS metastases, and the use of trastuzumab in the adjuvant setting does not prevent brain metastases. The purpose of this study is to compare outcomes in HER2-positive and HER2-negative intracranial disease treated with stereotactic radiosurgery (SRS). Among 57 breast cancer patients with brain metastases, 28 patients were HER2-positive. All patients were treated with SRS as their first treatment modality for CNS metastases. The median dose was 20 Gy (range 12–20 Gy). Statistical analysis was performed using the Kaplan–Meier method and χ 2 test. With a median follow-up of 11.0 months, the median time to progression in the HER2-positive group compared with the HER2-negative group was 7 versus 11 months (p = 0.080), respectively. Salvage therapy was performed in 50 % of HER2-positive patients compared with 21 % of HER2-negative patients (p = 0.02). The median OS for the HER2-positive group compared with the HER2-negative group was 22 versus 12 months (p = 0.053). Stereotactic radiosurgery results in excellent local control in the treatment for breast cancer brain metastases. Compared with HER2-negative disease, HER2-positive disease appears to show higher rates of intracranial relapse despite better overall survival rates. This data suggests that we need effective adjuvant therapy to prevent and treat brain metastases in HER2-positive patients.


Similar content being viewed by others
References
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi:10.3322/caac.21149.
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973–2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–72. doi:10.1200/JCO.2004.12.149.
Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101(4):810–6. doi:10.1002/cncr.20418.
Slimane K, Andre F, Delaloge S, Dunant A, Perez A, Grenier J, et al. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol. 2004;15(11):1640–4. doi:10.1093/annonc/mdh432.
Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107(4):696–704. doi:10.1002/cncr.22041.
Xue J, Peng G, Yang JS, Ding Q, Cheng J. Predictive factors of brain metastasis in patients with breast cancer. Med Oncol. 2013;30(1):337. doi:10.1007/s12032-012-0337-2.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi:10.1056/NEJMoa052122.
Bria E, Cuppone F, Fornier M, Nistico C, Carlini P, Milella M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109(2):231–9. doi:10.1007/s10549-007-9663-z.
Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–43. doi:10.1158/1078-0432.CCR-10-2962.
Soffietti R, Ruda R, Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol. 2008;20(6):676–84. doi:10.1097/CCO.0b013e32831186fe.
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–51.
Horton JK, Halle J, Ferraro M, Carey L, Moore DT, Ollila D, et al. Radiosensitization of chemotherapy-refractory, locally advanced or locally recurrent breast cancer with trastuzumab: a phase II trial. Int J Radiat Oncol Biol Phys. 2010;76(4):998–1004. doi:10.1016/j.ijrobp.2009.03.027.
Sartor CI. Epidermal growth factor family receptors and inhibitors: radiation response modulators. Semin Radiat Oncol. 2003;13(1):22–30. doi:10.1053/srao.2003.50003.
Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–80.
Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278–86. doi:10.1200/JCO.2008.19.8481.
Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013. doi:10.1016/S1470-2045(13)70017-2.
Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13–22. doi:10.1007/s10549-013-2525-y.
Lin NU. Brain metastases in HER2-positive breast cancer. Lancet Oncol. 2013. doi:10.1016/S1470-2045(13)70046-9.
Acknowledgments
This study was partially funded by the New York University Cancer Institute.
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tam, M., Narayana, A., Raza, S. et al. Role of HER2 status in the treatment for brain metastases arising from breast cancer with stereotactic radiosurgery. Med Oncol 31, 832 (2014). https://doi.org/10.1007/s12032-013-0832-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0832-0