Abstract
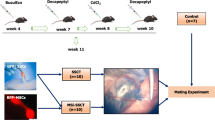
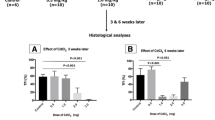
Very small embryonic-like stem cells (VSELs) exist among spermatogonial stem cells and survive chemotherapy in both mice and human testes because of their relatively quiescent nature. Our earlier study revealed that inter-tubular transplantation of niche (Sertoli or bone marrow derived mesenchymal) cells can restore spermatogenesis from endogenous surviving VSELs. Present study was undertaken to delineate the effect of busulphan on testicular stem/germ/Sertoli cells and to comprehend the underlying mechanisms of how transplanted niche cells restore spermatogenesis. Ploidy analysis showed an increase in diploid cells on D7 and VSELs (2–6 μm; LIN−/CD45−/SCA-1+) were detected at all time-points studied and were maximum on D15 after busulphan treatment. They were visualized in cell smears, expressed nuclear NANOG and SOX2 and BrdU uptake on D15 suggested they were proliferating in response to stress induced by busulphan. Verapamil-sensitive side population detected comprised SCA-1 positive stem cells (5 ± 0.02 % in normal and 8.6 ± 2.02 % in chemoablated testis). Adverse effects of busulphan on Sertoli cells by transcriptome analysis included altered expression of Gdnf, Scf, Fgf, Bmp4, androgen binding protein, components of blood-testis-barrier and also stem cells related signaling pathways including Wnt. GFP positive transplanted cells aligned themselves as ‘neo-tubules’ and were visualized adjacent to ‘native’ germ cells depleted tubules. ‘Neo-tubules’ provide paracrine support to endogenous VSELs to undergo spermatogenesis. Quantitative analysis was done to track proliferation (PCNA) and differentiation (MVH) of stem cells by immuno-localization studies at different time intervals. Results provide an alternative strategy to restore spermatogenesis in cancer survivors from endogenous stem cells which needs to be further researched.













Similar content being viewed by others
References
Bhartiya, D., Kasiviswanathan, S., Unni, S. K., et al. (2010). Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. The Journal of Histochemistry and Cytochemistry, 58(12), 1093–1106.
Kurkure, P., Prasad, M., Dhamankar, V., & Bakshi, G. (2015). Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reproductive Biology and Endocrinology, 13, 122.
Stimpfel, M., Skutella, T., Kubista, M., Malicev, E., Conrad, S., & Virant-Klun, I. (2012). Potential stemness of frozen-thawed testicular biopsies without sperm in infertile men included into the in vitro fertilization programme. Journal of Biomedicine & Biotechnology, 2012, 291038.
Virant-Klun, I., Stimpfel, M., Cvjeticanin, B., Vrtacnik-Bokal, E., & Skutella, T. (2013). Small SSEA-4-positive cells from human ovarian cell cultures: related to embryonic stem cells and germinal lineage? J Ovarian Res, 6, 24.
Anand, S., Bhartiya, D., Sriraman, K., Patel, H., & Manjramkar, D. D. (2014). Very small embryonic-like stem cells survive and restore spermatogenesis after busulphan treatment in mouse testis. J Stem Cell Res Ther, 4, 216.
Patel H, Bhartiya D. (2016). Testicular stem cells express follicle stimulating hormone receptors and are directly modulated by FSH. Reproductive Sciences, (in press). pii: 1933719116643593.
Anand, S., Patel, H., & Bhartiya, D. (2015). Chemoablated mouse seminiferous tubular cells enriched for very small embryonic-like stem cells undergo spontaneous spermatogenesis in vitro. Reproductive Biology and Endocrinology, 13, 33.
Zhang, Z., Shao, S., & Meistrich, M. L. (2007). The radiation induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. Journal of Cellular Physiology, 211(1), 149–158.
Shinohara, T., Orwig, K. E., Avarbock, M. R., & Brinster, R. L. (2003). Restoration of spermatogenesis in infertile mice by sertoli cell transplantation. Biology of Reproduction, 68(3), 1064–1071.
Kanatsu-Shinohara, M., Miki, H., Inoue, K., et al. (2005). Germline niche transplantation restores fertility in infertile mice. Human Reproduction, 20(9), 2376–2382.
Kanatsu-Shinohara, M., Morimoto, H., & Shinohara, T. (2016). Fertility of male germ line stem cells following spermatogonial transplantation in infertile mouse models. Biology of Reproduction, 94(5), 112.
Zhang, Z., Shao, S., Shetty, G., & Meistrich, M. L. (2009). Donor sertoli cells transplanted into irradiated rat testes stimulate partial recovery of endogenous spermatogenesis. Reproduction, 137(3), 497–508.
Ogawa, T., Dobrinski, I., & Brinster, R. L. (1999). Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue & Cell, 31(5), 461–472.
Challen, G. A., & Little, M. H. (2006). A side order of stem cells: the SP phenotype. Stem Cells, 24(1), 3–12.
Gavish, Z., Peer, G., Roness, H., Cohen, Y., & Meirow, D. (2014). Follicle activation and 'burn-out' contribute to post-transplantation follicle loss in ovarian tissue grafts: the effect of graft thickness. Human Reproduction, 29(5), 989–996.
Kucia, M., Reca, R., Campbell, F. R., et al. (2006). A population of very small embryonic-like (VSEL) CXCR4(+) SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia, 20(5), 857–869.
Shimizu, Y., Motohashi, N., Iseki, H., Kunita, S., Sugiyama, F., & Yagami, K. (2006). A novel subpopulation lacking Oct4 expression in the testicular side population. International Journal of Molecular Medicine, 17(1), 21–28.
Kubota, H., Avarbock, M. R., & Brinster, R. L. (2003). Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proceedings of the National Academy of Sciences of the United States of America, 100(11), 6487–6492.
Falciatori, I., Borsellino, G., Haliassos, N., et al. (2004). Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. The FASEB Journal, 18(2), 376–378.
Chen, S., Lewallen, M., & Xie, T. (2013). Adhesion in the stem cell niche: biological roles and regulation. Development, 140(2), 255–265.
Boyer, A., Hermo, L., Paquet, M., Robaire, B., & Boerboom, D. (2008). Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in sertoli cells. Biology of Reproduction, 79(3), 475–485.
vanBragt, M. P., Ciliberti, N., Stanford, W. L., de Rooij, D. G., & van Pelt, A. M. (2005). LY6A/E (SCA-1) expression in the mouse testis. Biology of Reproduction, 73(4), 634–638.
Walker, W. H., & Cheng, J. (2005). FSH and testosterone signaling in sertoli cells. Reproduction, 130(1), 15–28.
Boyer, A., Yeh, J. R., Zhang, X., et al. (2012). CTNNB1 signaling in sertoli cells down regulates spermatogonial stem cell activity via WNT4. PloS One, 7(1), e29764.
Tanwar, P. S., Kaneko-Tarui, T., Zhang, L., Rani, P., Taketo, M. M., & Teixeira, J. (2010). Constitutive WNT/beta-catenin signaling in murine sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biology of Reproduction, 82(2), 422–432.
Ryu, B. Y., Orwig, K. E., Oatley, J. M., Avarbock, M. R., & Brinster, R. L. (2006). Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells, 24(6), 1505–1511.
Russell, L. D., & Griswold, M. D. (2000). Spermatogonial transplantation-an update for the millennium. Molecular and Cellular Endocrinology, 161(1–2), 117–120.
Ramathal, C., Durruthy-Durruthy, J., Sukhwani, M., et al. (2014). Fate of iPSCs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Reports, 7(4), 1284–1297.
Forbes, S. J., & Rosenthal (2014). Preparing the ground for tissue regeneration: From mechanism to therapy. Nature Medicine, 20(8), 857–869.
Bhartiya, D., Shaikh, A., Anand, S., Patel, H., Kapoor, H., Sriraman, K., Parte, S., & Unni, S. (2016) Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Human Reproduction Update. doi:10.1093/humupd/dmw030
Acknowledgments
We thank Harshada Modak and Smita Bhutda from the Stem Cell Biology Department for technical help. The help from Dr. Mukherjee, Ms. Gayatri and Ms. Sushma in flow cytometry studies and from Ms. Reshma and Ms. Shobha in confocal studies is also acknowledged. We also thank Indian Council of Medical Research and Department of Science and Technology, Government of India, New Delhi for providing financial support for the study.
Author Contributions
SA Conception and design, Collection of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript; DB : Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript KS: Collection of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript. AM: Collection of data, Final approval of manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimers
Authors declare no conflict of interest.
Funding
Financial Support for the study was provided by Indian Council of Medical Research and Department of Science and Technology, Government of India, New Delhi, India.
Electronic supplementary material
ESM 1
(PDF 5418 kb)
Rights and permissions
About this article
Cite this article
Anand, S., Bhartiya, D., Sriraman, K. et al. Underlying Mechanisms that Restore Spermatogenesis on Transplanting Healthy Niche Cells in Busulphan Treated Mouse Testis. Stem Cell Rev and Rep 12, 682–697 (2016). https://doi.org/10.1007/s12015-016-9685-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9685-1