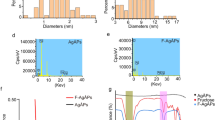
Abstract
The aim of the study was to evaluate if gold-coated superparamagnetic iron oxide nanoparticles (AuSPION) magnetic-targeted to the arthritic articulation of collagen induced arthritis (CIA) rats are able to ameliorate rheumatoid arthritis without producing significant biological adverse effects in comparison to colloidal Au nanoparticles (AuC) and metotrexate (MTX). Male Wistar rats were divided into control; arthritic; AuSPION (150 μg kg−1); AuC (150 μg kg−1) and MTX (2.5 μg kg−1). Treatments were administered thrice every other day by the intraperitoneal route 15 min after all groups had a neodymium magnet coupled to the right ankle joint (kept for 1 h). Paw edema and body weight were measured weekly. Joint sections were evaluated by Haematoxylin & Eosin and immunohistochemistry (TNF-α, IL-1β). Biomarkers of oxidative stress were used to evaluate toxicity. Among the evaluated treatments, AuSPION led to significant clinical improvements (decreased edema and infiltration by leukocytes as well as less positively immunostained cells for both TNF-α and IL-1β in synovium) accompanied by a lack of toxicity as indicated by redox state and genotoxicity assays. Our results clearly indicate that the magnetic targeting of AuSPION suppresses joint edema and inflammation, cytokine expression as well as the redox imbalance, thereby contributing to an amelioration of arthritis severity in CIA rats. The results demonstrate for the first time the potentiality of AuSPION administration under a magnetic field as an attractive alternative for future treatments of rheumatic diseases.





Similar content being viewed by others
References
Adebayo OA, Akinloye O,Adaramoye OA (2019) Cerium oxide nanoparticles attenuate oxidative stress and inflammation in the liver of Diethylnitrosamine-treated mice. Biol Trace Elem Res
Suh KS, Lee YS, Seo SH, Kim YS, Choi EM (2013) Gold nanoparticles attenuates antimycin A-induced mitochondrial dysfunction in MC3T3-E1 osteoblastic cells. Biol Trace Elem Res 153(1–3):428–436
Sisubalan N, Ramkumar VS, Pugazhendhi A, Karthikeyan C, Indira K, Gopinath K, Hameed ASH, Basha MHG (2018) ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ Sci Pollut Res Int 25(11):10482–10492
Nagarajan D, Venkatanarasimhan S (2019) Copper (II) oxide nanoparticles coated cellulose sponge-an effective heterogeneous catalyst for the reduction of toxic organic dyes
Jia Z, Lyu F, Zhang LC, Zeng S, Liang SX, Li YY, Lu J (2019) Pt nanoparticles decorated heterostructured g-C3N4/Bi2MoO6 microplates with highly enhanced photocatalytic activities under visible light. Environ Sci Pollut Res Int 9(1):7636
Suganthy N, Sri Ramkumar V, Pugazhendhi A, Benelli G, Archunan G (2018) Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ Sci Pollut Res Int 25(11):10418–10433
Liu Y, Jie X, Guo Y, Zhang X, Wang J, Xue C (2016) Green synthesis of Oxovanadium(IV)/chitosan nanocomposites and its ameliorative effect on hyperglycemia, insulin resistance, and oxidative stress. Biol Trace Elem Res 169(2):310–319
Vasantharaj S, Sathiyavimal S, Senthilkumar P, LewisOscar F, Pugazhendhi A (2019) Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: antimicrobial properties and their applications in photocatalytic degradation. J Photochem Photobiol B 192:74–82
Vasantharaj S, Sathiyavimal S, Saravanan M, Senthilkumar P, Gnanasekaran K, Shanmugavel M, … Pugazhendhi A (2019) Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B 191:143–149
Sathiyavimal S, Vasantharaj S, Bharathi D, Saravanan M, Manikandan E, Kumar SS, Pugazhendhi A (2018) Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of gram negative and gram positive bacteria. J Photochem Photobiol B 188:126–134
Fathima JB, Pugazhendhi A, Venis R (2017) Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb Pathog 110:245–251
Valavanidis AVlachogianni T (2016) Engineered nanomaterials for pharmaceutical and biomedical products new trends, benefits and opportunities. Pharm Bioprocess 4(1)
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055):2023–2038
Strand VKhanna D (2010) The impact of rheumatoid arthritis and treatment on patients' lives. Clin Exp Rheumatol 28(3 Suppl 59):S32–S40
Thakor AS, Jokerst J, Zavaleta C, Massoud TF, Gambhir SS (2011) Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett 11(10):4029–4036
Sarzi-Puttini P, Ceribelli A, Marotto D, Batticciotto A, Atzeni F (2019) Systemic rheumatic diseases: from biological agents to small molecules. Autoimmun Rev 18(6):583–592
Calasan MB, van den Bosch OF, Creemers MC, Custers M, Heurkens AH, van Woerkom JM, Wulffraat NM (2013) Prevalence of methotrexate intolerance in rheumatoid arthritis and psoriatic arthritis. Arthritis Res Ther 15(6):R217
Bryant PA, Baddley JW (2017) Opportunistic infections in biological therapy, risk and prevention. Rheum Dis Clin N Am 43(1):27–41
Tsai CY, Shiau AL, Chen SY, Chen YH, Cheng PC, Chang MY, Chen DH, Chou CH, Wang CR, Wu CL (2007) Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum 56(2):544–554
Huang YJ, Shiau AL, Chen SY, Chen YL, Wang CR, Tsai CY, … Wu CL (2012) Multivalent structure of galectin-1-nanogold complex serves as potential therapeutics for rheumatoid arthritis by enhancing receptor clustering. Eur Cell Mater 23:170–181; discussion 181
Ilinskaya AN, Dobrovolskaia MA (2014) Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol 171(17):3988–4000
Kim HJ, Lee SM, Park KH, Mun CH, Park YB, Yoo KH (2015) Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials 61:95–102
Brown KR, Walter DG, Natan M (2000) Seeding of colloidal au nanoparticle solutions. 2. Improved control for particle size and shape. Chem Mater 12:306–313
Lyon JL, Fleming DA, Stone MB, Schiffer P, Williams ME (2004) Synthesis of Fe oxide Core/au Shell nanoparticles by iterative hydroxylamine seeding. Nano Lett 4:719–723
Hu Y, Cheng W, Cai W, Yue Y, Li J, Zhang P (2013) Advances in research on animal models of rheumatoid arthritis. Clin Rheumatol 32(2):161–165
Lindh I, Snir O, Lonnblom E, Uysal H, Andersson I, Nandakumar KS, … Holmdahl R (2014) Type II collagen antibody response is enriched in the synovial fluid of rheumatoid joints and directed to the same major epitopes as in collagen induced arthritis in primates and mice. Arthritis Res Ther 16(4):R143
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Passaglia P, Ceron CS, Mecawi AS, Antunes-Rodrigues J, Coelho EB, Tirapelli CR (2015) Angiotensin type 1 receptor mediates chronic ethanol consumption-induced hypertension and vascular oxidative stress. Vasc Pharmacol 74:49–59
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175(1):184–191
da Silva J, de Freitas TR, Heuser V, Marinho JR, Erdtmann B (2000) Genotoxicity biomonitoring in coal regions using wild rodent Ctenomys torquatus by comet assay and micronucleus test. Environ Mol Mutagen 35(4):270–278
Hayashi M, Morita T, Kodama Y, Sofuni T, Ishidate M Jr (1990) The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat Res 245(4):245–249
Carneiro-Filho BA, Lima IP, Araujo DH, Cavalcante MC, Carvalho GH, Brito GA, … Lima AA (2004) Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig Dis Sci 49(1):65–72
Leonaviciene L, Kirdaite G, Bradunaite R, Vaitkiene D, Vasiliauskas A, Zabulyte D, … Mackiewicz Z (2012) Effect of gold nanoparticles in the treatment of established collagen arthritis in rats. Medicina (Kaunas) 48(2):91–101
Sumbayev VV, Yasinska IM, Garcia CP, Gilliland D, Lall GS, Gibbs BF, Bonsall DR, Varani L, Rossi F, Calzolai L (2013) Gold nanoparticles downregulate interleukin-1beta-induced pro-inflammatory responses. Small 9(3):472–477
Malaczewska J (2014) The in vitro effect of commercially available noble metal nanocolloids on the splenocyte proliferative response and cytokine production in mice. Pol J Vet Sci 17(1):37–45
Han Z, Boyle DL, Manning AM, Firestein GS (1998) AP-1 and NF-kappaB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity 28(4):197–208
Gul A, Kunwar B, Mazhar M, Faizi S, Ahmed D, Shah MR, Simjee SU (2018) Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-kappaB and iNOS activation. Int Immunopharmacol 59:310–317
von der Weid PY, Crowe MJ, Van Helden DF (1996) Endothelium-dependent modulation of pacemaking in lymphatic vessels of the Guinea-pig mesentery. J Physiol 493(Pt 2):563–575
Palma Zochio Tozzato G, Taipeiro EF, Spadella MA, Marabini Filho P, de Assis MR, Carlos CP, Girol AP, Chies AB (2016) Collagen-induced arthritis increases inducible nitric oxide synthase not only in aorta but also in the cardiac and renal microcirculation of mice. Clin Exp Immunol 183(3):341–349
Cannon GW, Openshaw SJ, Hibbs JB Jr, Hoidal JR, Huecksteadt TP, Griffiths MM (1996) Nitric oxide production during adjuvant-induced and collagen-induced arthritis. Arthritis Rheum 39(10):1677–1684
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75(6):639–653
Umar S, Zargan J, Umar K, Ahmad S, Katiyar CK, Khan HA (2012) Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem Biol Interact 197(1):40–46
Taysi S, Polat F, Gul M, Sari RA, Bakan E (2002) Lipid peroxidation, some extracellular antioxidants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol Int 21(5):200–204
Remans PH, van Oosterhout M, Smeets TJ, Sanders M, Frederiks WM, Reedquist KA, … van Laar JM (2005) Intracellular free radical production in synovial T lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum 52(7):2003–2009
Kurien BT, Scofield RH (2008) Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev 7(7):567–573
Jeon CH, Ahn JK, Chai JY, Kim HJ, Bae EK, Park SH et al (2008) Hypoxia appears at pre-arthritic stage and shows co-localization with early synovial inflammation in collagen induced arthritis. Clin Exp Rheumatol 26(4):646–648
Biniecka M, Kennedy A, Fearon U, Ng CT, Veale DJ, O'Sullivan JN (2010) Oxidative damage in synovial tissue is associated with in vivo hypoxic status in the arthritic joint. Ann Rheum Dis 69(6):1172–1178
Sul OJ, Kim JC, Kyung TW, Kim HJ, Kim YY, Kim SH et al (2010) Gold nanoparticles inhibited the receptor activator of nuclear factor-kappab ligand (RANKL)-induced osteoclast formation by acting as an antioxidant. Biosci Biotechnol Biochem 74(11):2209–2213
Dkhil MA, Bauomy AA, Diab MS, Al-Quraishy S (2015) Antioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasis. Int J Nanomedicine 10:7467–7475
Kirdaite G, Leonaviciene L, Bradunaite R, Vasiliauskas A, Rudys R, Ramanaviciene A, Mackiewicz Z (2019) Antioxidant effects of gold nanoparticles on early stage of collagen-induced arthritis in rats. Res Vet Sci 124:32–37
Khan MA, Subramaneyaan M, Arora VK, Banerjee BD, Ahmed RS (2015) Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J Complement Integr Med 12(2):117–125
Paula MM, Petronilho F, Vuolo F, Ferreira GK, De Costa L, Santos GP, … Pinho RA (2015) Gold nanoparticles and/or N-acetylcysteine mediate carrageenan-induced inflammation and oxidative stress in a concentration-dependent manner. J Biomed Mater Res A 103(10):3323–3330
Bednarski M, Dudek M, Knutelska J, Nowinski L, Sapa J, Zygmunt M, … Tesiorowski M (2015) The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: in vivo studies. Pharmacol Rep 67(3):405–409
Zhao Y, Gu X, Ma H, He X, Liu M, Ding Y (2011) Association of Glutathione Level and Cytotoxicity of gold nanoparticles in lung Cancer cells. J Phys Chem C 115(26):12797–12802
Tournebize J, Boudier A, Joubert O, Eidi H, Bartosz G, Maincent P, Leroy P, Sapin-Minet A (2012) Impact of gold nanoparticle coating on redox homeostasis. Int J Pharm 438(1–2):107–116
Giustarini D, Milzani A, Colombo R, Dalle-Donne I, Rossi R (2003) Nitric oxide and S-nitrosothiols in human blood. Clin Chim Acta 330(1–2):85–98
Jia HY, Liu Y, Zhang XJ, Han L, Du LB, Tian Q, Xu YC (2009) Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J Am Chem Soc 131(1):40–41
Sahu D, Raghav SK, Gautam H, Das HR (2015) A novel coumarin derivative, 8-methoxy chromen-2-one alleviates collagen induced arthritis by down regulating nitric oxide, NFkappaB and proinflammatory cytokines. Int Immunopharmacol 29(2):891–900
El-Sheikh AA, Morsy MA, Abdalla AM, Hamouda AH, Alhaider IA (2015) Mechanisms of Thymoquinone Hepatorenal protection in methotrexate-induced toxicity in rats. Mediat Inflamm 2015:859383
El-Sheikh AA, Morsy MA, Al-Taher AY (2016) Protective mechanisms of resveratrol against methotrexate-induced renal damage may involve BCRP/ABCG2. Fundam Clin Pharmacol 30(5):406–418
Armagan I, Bayram D, Candan IA, Yigit A, Celik E, Armagan HH, Uguz AC (2015) Effects of pentoxifylline and alpha lipoic acid on methotrexate-induced damage in liver and kidney of rats. Environ Toxicol Pharmacol 39(3):1122–1131
Iswarya V, Manivannan J, De A, Paul S, Roy R, Johnson JB et al (2016) Surface capping and size-dependent toxicity of gold nanoparticles on different trophic levels. Environ Sci Pollut Res Int 23(5):4844–4858
Conde J, Larguinho M, Cordeiro A, Raposo LR, Costa PM, Santos S, Diniz MS, Fernandes AR, Baptista PV (2014) Gold-nanobeacons for gene therapy: evaluation of genotoxicity, cell toxicity and proteome profiling analysis. Nanotoxicology 8(5):521–532
Downs TR, Crosby ME, Hu T, Kumar S, Sullivan A, Sarlo K, Reeder B, Lynch M, Wagner M, Mills T, Pfuhler S (2012) Silica nanoparticles administered at the maximum tolerated dose induce genotoxic effects through an inflammatory reaction while gold nanoparticles do not. Mutat Res 745(1–2):38–50
Balansky R, Longobardi M, Ganchev G, Iltcheva M, Nedyalkov N, Atanasov P, Toshkova R, de Flora S, Izzotti A (2013) Transplacental clastogenic and epigenetic effects of gold nanoparticles in mice. Mutat Res 751-752:42–48
Patel PShah J (2017) Safety and toxicological considerations of nanomedicines: the future directions. Curr Clin Pharmacol 12(2):73–82
Terentyuk GS, Maslyakova GN, Suleymanova LV, Khlebtsov BN, Kogan BY, Akchurin GG, Shantrocha AV, Maksimova IL, Khlebtsov NG, Tuchin VV (2009) Circulation and distribution of gold nanoparticles and induced alterations of tissue morphology at intravenous particle delivery. J Biophotonics 2(5):292–302
Arami H, Khandhar A, Liggitt D, Krishnan KM (2015) In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev 44(23):8576–8607
Iqbal MP, Mehboobali N, Sultana F, Khan FB, Burney IA, Kakepoto GN (2003) Effect of methotrexate and folinic acid on accumulation of iron in mice. Med Hypotheses 61(4):444–445
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160(7):1577–1579
Acknowledgements
The authors acknowledge the staff of Laboratório de Histologia da Faculdade de Odontologia de Ribeirão Preto (FORP/USP), Laboratório de Farmacologia do Instituto Butantan and Laboratório de Neuroendocrinologia da Faculdade de Medicina de Ribeirão Preto (USP) and to Ana Flavia do Bem Afonso for technical support. Authors are grateful to Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) for financial support (FAPESP 2014/06744-4 and FAPESP 2015/0538-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing financial or non-financial interests. The results of this study were obtained in compliance with the NC3Rs ARRIVE guidelines [73]. Procedures were developed also in accordance with the guidelines of the Committee on the Care and Use of Experimental Animal Resources of the University of Sao Paulo after the approval of the study by the same Committee (opinion number: 14.1.550.53.7).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 150 kb)
Rights and permissions
About this article
Cite this article
Carneiro, M.H., Machado, A.R.T., Antunes, L.M.G. et al. Gold-Coated Superparamagnetic Iron Oxide Nanoparticles Attenuate Collagen-Induced Arthritis after Magnetic Targeting. Biol Trace Elem Res 194, 502–513 (2020). https://doi.org/10.1007/s12011-019-01799-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01799-z