Abstract
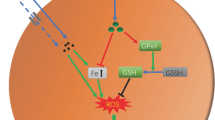
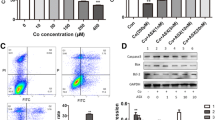
Currently, tissue damage induced by cobalt nanoparticles (CoNPs) and cobalt ions (Co2+) are the most serious syndrome in the patients with metal-on-metal hip prostheses. Therefore, an urgent need exists for the identification of the mechanisms and the development of therapeutic strategies to limit it. The purpose of this study was to explore the mechanism of this damage and to demonstrate if l-ascorbic acid (L-AA) could protect against the cell toxicities induced by CoNPs and Co2+ in vitro. With CoNPs and Co2+ treatment, cell viability was significantly decreased; the ROS (reactive oxygen species) level in mitochondria was dramatically increased in CoNPs treated cells, but cobalt ions could barely induce the ROS. Consistently, the level of cell apoptosis was increased with the upregulation of pro-apoptotic factors (caspases 8, 9, and 3, and Bax) and the downregulation of anti-apoptotic factor Bcl-2. Besides that, the levels of cytochrome c and AIF were increased and released from mitochondria into the cytoplasm. After the cells were pretreated with L-AA, the cell viability decreased by CoNPs was reversed and the ROS induced by CoNPs was suppressed. The level of cell apoptosis induced by CoNPs was decreased as well. But it could not reverse the effects induced by Co2+. These studies demonstrated that CoNPs induce extrinsic and intrinsic apoptotic pathways via generation of ROS, and L-AA could prevent the cytotoxicity by reducing the level of ROS. While Co2+ may induce cytotoxicity through other signals, it could not be protected by L-AA treatment.








Similar content being viewed by others
Abbreviations
- CoNPs:
-
Cobalt nanoparticles
- Co2+ :
-
Cobalt ions
- TEM:
-
Transmission electron microscopy
- SEM:
-
Scanning electron microscope
- MOM:
-
Metal-on-metal
- L-AA:
-
l-Ascorbic acid
- ROS:
-
Reactive oxygen species
- Caspase:
-
Cysteinyl aspartate specific proteinase
- IARC:
-
International Agency for Research on Cancer
- GSH:
-
Glutathione
References
Gill HS, Grammatopoulos G, Adshead S, Tsialogiannis E, Tsiridis E (2012) Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol Med 18(3):145–155. doi:10.1016/j.molmed.2011.12.002
Xia Z, Kwon YM, Mehmood S, Downing C, Jurkschat K, Murray DW (2011) Characterization of metal-wear nanoparticles in pseudotumor following metal-on-metal hip resurfacing. Nanomed : Nanotechnol Biol Med 7(6):674–681. doi:10.1016/j.nano.2011.08.002
Saikko V, Nevalainen J, Revitzer H, Ylinen P (1998) Metal release from total hip articulations in vitro: substantial from CoCr/CoCr, negligible from CoCr/PE and alumina/PE. Acta Orthop Scand 69(5):449–454
Milosev I, Remskar M (2009) In vivo production of nanosized metal wear debris formed by tribochemical reaction as confirmed by high-resolution TEM and XPS analyses. J Biomed Mater Res Part A 91(4):1100–1110. doi:10.1002/jbm.a.32301
Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K (2007) The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med 64(9):609–615. doi:10.1136/oem.2005.024802
Ponti J, Sabbioni E, Munaro B, Broggi F, Marmorato P, Franchini F, Colognato R, Rossi F (2009) Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: an in vitro study in Balb/3T3 mouse fibroblasts. Mutagenesis 24(5):439–445. doi:10.1093/mutage/gep027
Colognato R, Bonelli A, Ponti J, Farina M, Bergamaschi E, Sabbioni E, Migliore L (2008) Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis 23(5):377–382. doi:10.1093/mutage/gen024
McGregor DB, Baan RA, Partensky C, Rice JM, Wilbourn JD (2000) Evaluation of the carcinogenic risks to humans associated with surgical implants and other foreign bodies—a report of an IARC monographs Programme meeting. International Agency for Research on Cancer. Eur J Cancer 36(3):307–313
Jiang H, Liu F, Yang H, Li Y (2012) Effects of cobalt nanoparticles on human T cells in vitro. Biol Trace Elem Res 146(1):23–29. doi:10.1007/s12011-011-9221-8
Lombaert N, De Boeck M, Decordier I, Cundari E, Lison D, Kirsch-Volders M (2004) Evaluation of the apoptogenic potential of hard metal dust (WC-Co), tungsten carbide and metallic cobalt. Toxicol Lett 154(1–2):23–34. doi:10.1016/j.toxlet.2004.06.009
Lombaert N, Lison D, Van Hummelen P, Kirsch-Volders M (2008) In vitro expression of hard metal dust (WC-Co)—responsive genes in human peripheral blood mononucleated cells. Toxicol Appl Pharmacol 227(2):299–312. doi:10.1016/j.taap.2007.11.002
Ding M, Kisin ER, Zhao J, Bowman L, Lu Y, Jiang B, Leonard S, Vallyathan V, Castranova V, Murray AR, Fadeel B, Shvedova AA (2009) Size-dependent effects of tungsten carbide-cobalt particles on oxygen radical production and activation of cell signaling pathways in murine epidermal cells. Toxicol Appl Pharmacol 241(3):260–268. doi:10.1016/j.taap.2009.09.004
Zhao J, Bowman L, Magaye R, Leonard SS, Castranova V, Ding M (2013) Apoptosis induced by tungsten carbide-cobalt nanoparticles in JB6 cells involves ROS generation through both extrinsic and intrinsic apoptosis pathways. Int J Oncol 42(4):1349–1359. doi:10.3892/ijo.2013.1828
Sabbioni E, Fortaner S, Farina M, Del Torchio R, Olivato I, Petrarca C, Bernardini G, Mariani-Costantini R, Perconti S, Di Giampaolo L, Gornati R, Di Gioacchino M (2014) Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology 8(4):455–464. doi:10.3109/17435390.2013.796538
Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP (2007) The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials 28(19):2946–2958. doi:10.1016/j.biomaterials.2007.02.034
Corvi R, Aardema MJ, Gribaldo L, Hayashi M, Hoffmann S, Schechtman L, Vanparys P (2012) ECVAM prevalidation study on in vitro cell transformation assays: general outline and conclusions of the study. Mutat Res 744(1):12–19. doi:10.1016/j.mrgentox.2011.11.009
Nyga A, Hart A, Tetley TD (2015) Importance of the HIF pathway in cobalt nanoparticle-induced cytotoxicity and inflammation in human macrophages. Nanotoxicology 9(7):905–917. doi:10.3109/17435390.2014.991430
Tao S, Zheng Y, Lau A, Jaramillo MC, Chau BT, Lantz RC, Wong PK, Wondrak GT, Zhang DD (2013) Tanshinone I activates the Nrf2-dependent antioxidant response and protects against As(III)-induced lung inflammation in vitro and in vivo. Antioxid Redox Signal 19(14):1647–1661. doi:10.1089/ars.2012.5117
Nguyen VT, Ko SC, Oh GW, Heo SY, Jeon YJ, Park WS, Choi IW, Choi SW, Jung WK (2016) Anti-inflammatory effects of sodium alginate/gelatine porous scaffolds merged with fucoidan in murine microglial BV2 cells. Int J Biol Macromol. doi:10.1016/j.ijbiomac.2016.05.078
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1(6):3159–3165. doi:10.1038/nprot.2006.378
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627. doi:10.1126/science.1114397
Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, Ryter SW, Choi AM (2008) Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy 4(7):887–895
Paolicchi A, Dominici S, Pieri L, Maellaro E, Pompella A (2002) Glutathione catabolism as a signaling mechanism. Biochem Pharmacol 64(5–6):1027–1035
Oguro T, Hayashi M, Nakajo S, Numazawa S, Yoshida T (1998) The expression of heme oxygenase-1 gene responded to oxidative stress produced by phorone, a glutathione depletor, in the rat liver; the relevance to activation of c-Jun n-terminal kinase. J Pharmacol Exp Ther 287(2):773–778
Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prevost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G (2000) Two distinct pathways leading to nuclear apoptosis. J Exp Med 192(4):571–580
Campbell P, Shimmin A, Walter L, Solomon M (2008) Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplast 23(7):1080–1085. doi:10.1016/j.arth.2007.09.024
Hart AJ, Skinner JA, Winship P, Faria N, Kulinskaya E, Webster D, Muirhead-Allwood S, Aldam CH, Anwar H, Powell JJ (2009) Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J Bone Joint Surg Br Vol 91(6):835–842. doi:10.1302/0301-620X.91B6.21844
Huang DC, Tatman P, Mehle S, Gioe TJ (2013) Cumulative revision rate is higher in metal-on-metal THA than metal-on-polyethylene THA: analysis of survival in a community registry. Clin Orthop Relat Res 471(6):1920–1925. doi:10.1007/s11999-013-2821-1
Cirla AM (1994) Cobalt-related asthma: clinical and immunological aspects. Sci Total Environ 150(1–3):85–94
Lison D, Lauwerys R, Demedts M, Nemery B (1996) Experimental research into the pathogenesis of cobalt/hard metal lung disease. Eur Respir J 9(5):1024–1028
Hart AJ, Quinn PD, Lali F, Sampson B, Skinner JA, Powell JJ, Nolan J, Tucker K, Donell S, Flanagan A, Mosselmans JF (2012) Cobalt from metal-on-metal hip replacements may be the clinically relevant active agent responsible for periprosthetic tissue reactions. Acta Biomater 8(10):3865–3873. doi:10.1016/j.actbio.2012.05.003
Perumal V, Alkire M, Swank ML (2010) Unusual presentation of cobalt hypersensitivity in a patient with a metal-on-metal bearing in total hip arthroplasty. Am J Orthop 39(5):E39–E41
Black J (1984) Systemic effects of biomaterials. Biomaterials 5(1):11–18
Dillon CT, Lay PA, Bonin AM, Cholewa M, Legge GJ (2000) Permeability, cytotoxicity, and genotoxicity of Cr(III) complexes and some Cr(V) analogues in V79 Chinese hamster lung cells. Chem Res Toxicol 13(8):742–748
Papis E, Gornati R, Prati M, Ponti J, Sabbioni E, Bernardini G (2007) Gene expression in nanotoxicology research: analysis by differential display in BALB3T3 fibroblasts exposed to cobalt particles and ions. Toxicol Lett 170(3):185–192. doi:10.1016/j.toxlet.2007.03.005
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6(8):1794–1807. doi:10.1021/nl061025k
Avalos A, Haza AI, Mateo D, Morales P (2014) Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J Appl Toxicol : JAT 34(4):413–423. doi:10.1002/jat.2957
Park EJ, Yi J, Kim Y, Choi K, Park K (2010) Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol In Vitro : Int J Published Assoc BIBRA 24(3):872–878. doi:10.1016/j.tiv.2009.12.001
Battaglia V, Compagnone A, Bandino A, Bragadin M, Rossi CA, Zanetti F, Colombatto S, Grillo MA, Toninello A (2009) Cobalt induces oxidative stress in isolated liver mitochondria responsible for permeability transition and intrinsic apoptosis in hepatocyte primary cultures. Int J Biochem Cell Biol 41(3):586–594. doi:10.1016/j.biocel.2008.07.012
Caroppi P, Sinibaldi F, Fiorucci L, Santucci R (2009) Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome C as proapoptotic protein. Curr Med Chem 16(31):4058–4065
Yang W, Shi L, Chen L, Zhang B, Ma K, Liu Y, Qian Y (2014) Protective effects of perindopril on d-galactose and aluminum trichloride induced neurotoxicity via the apoptosis of mitochondria-mediated intrinsic pathway in the hippocampus of mice. Brain Res Bull 109:46–53. doi:10.1016/j.brainresbull.2014.09.010
Siskind LJ, Kolesnick RN, Colombini M (2006) Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 6(3):118–125. doi:10.1016/j.mito.2006.03.002
Landshamer S, Hoehn M, Barth N, Duvezin-Caubet S, Schwake G, Tobaben S, Kazhdan I, Becattini B, Zahler S, Vollmar A, Pellecchia M, Reichert A, Plesnila N, Wagner E, Culmsee C (2008) Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ 15(10):1553–1563. doi:10.1038/cdd.2008.78
Garland JM, Rudin C (1998) Cytochrome c induces caspase-dependent apoptosis in intact hematopoietic cells and overrides apoptosis suppression mediated by bcl-2, growth factor signaling, MAP-kinase-kinase, and malignant change. Blood 92(4):1235–1246
Nyga A, Hart A, Tetley TD (2015) Importance of the HIF pathway in cobalt nanoparticle-induced cytotoxicity and inflammation in human macrophages. Nanotoxicology:1–13. doi:10.3109/17435390.2014.991430
Chairuangkitti P, Lawanprasert S, Roytrakul S, Aueviriyavit S, Phummiratch D, Kulthong K, Chanvorachote P, Maniratanachote R (2013) Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol In Vitro: Int J Published Assoc BIBRA 27(1):330–338. doi:10.1016/j.tiv.2012.08.021
Acknowledgment
This study was funded by the National Natural Science Foundation of China (No. 81171743) and Jiangsu Province Natural Science Foundation of China (No. BK20150399).
Authors’ Contributions
YKL wrote the manuscript and designed the study, HXH conducted the experimental work and designed the study, XL and WW performed the experimental work and statistical analysis, FL supervised the project and conducted the statistical analysis, and HLY was responsible for the whole project and supervised the study. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no declarations of interest.
Additional information
Yake Liu and Hongxiang Hong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Hong, H., Lu, X. et al. l-Ascorbic Acid Protected Against Extrinsic and Intrinsic Apoptosis Induced by Cobalt Nanoparticles Through ROS Attenuation. Biol Trace Elem Res 175, 428–439 (2017). https://doi.org/10.1007/s12011-016-0789-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0789-x