Abstract
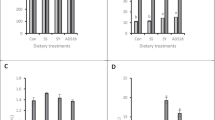
In geographic regions where selenium (Se) soil concentrations are naturally low, the addition of Se to animal feed is necessary. Even though it is known that Se in grass and forage crops is primarily present in organic forms (especially as L-selenomethionine, L-selenocystine, and L-selenocystathionine), the feeding of Se in the naturally occurring organic selenium (OSe) compounds produces higher blood and tissue Se levels than the inorganic Se (ISe) salts, and that animal metabolism of OSe and ISe is fundamentally different. Se is commonly added in inorganic form as sodium selenite to cattle feeds because it is a less expensive source of supplemental Se then are OSe forms. A trial was conducted with growing cattle to determine if the addition of OSe versus ISe forms of Se in beef cattle feed produces differences in hepatic gene expression, thereby gaining insight into the metabolic consequence of feeding OSe versus ISe. Thirty maturing Angus heifers (261 ± 6 days) were fed a corn silage-based diet with no Se supplementation for 75 days. Heifers (body weight = 393 ± 9 kg) then were randomly assigned (n = 10) and fed Se supplements that contained none (control) or 3 mg Se/day in ISe (sodium selenite) or OSe (Sel-Plex®) form and enough of a common cracked corn/cottonseed hull-based diet (0.48 mg Se/day) to support 0.5 kg/day growth for 105 or 106 days. More Se was found in jugular whole blood and red blood cells and biopsied liver tissue of ISe and OSe treatment animals than control animals, and OSe animals contained more Se in these tissues than did ISe. Microarray and bioinformatic analyses of liver tissue gene expression revealed that the content of at least 80 mRNA were affected by ISe or OSe treatments, including mRNA associated with nutrient metabolism; cellular growth, proliferation, and immune response; cell communication or signaling; and tissue/organ development and function. Overall, three Se supplement-dependent gene groups were identified: ISe-dependent, OSe-dependent, and Se form-independent. More specifically, both forms of supplementation appeared to upregulate mitochondrial gene expression capacity, whereas gene expression of a protein involved in antiviral capacity was downregulated in ISe-supplemented animals, and OSe-supplemented animals had reduced levels of mRNA encoding proteins known to be upregulated during oxidative stress and cancerous states.
Similar content being viewed by others
Abbreviations
- ANOVA:
-
analysis of variance
- CL-43:
-
collectin-43
- GCL:
-
glutamate-cysteine ligase
- GCLC:
-
glutamate-cysteine ligase catalytic subunit
- GCLM:
-
glutamate-cysteine ligase modifier subunit
- IGF:
-
insulin-like growth factor
- IGFBP:
-
insulin-like growth factor binding protein
- ISe:
-
inorganic selenium
- KLF:
-
Kruppel-like factor
- OSe:
-
organic selenium
- PRKCI:
-
protein kinase C iota
- Se:
-
selenium
References
Dargatz DA, Ross PF (1996) Blood selenium concentrations in cows and heifers on 253 cow-calf operations in 18 states. J Anim Sci 74:2891–2895
Pehrson BG (1993) Selenium in nutrition with special reference to the biopotency of organic and inorganic selenium compounds. In: Lyons TP (ed) Biotechnology in the Feed Industry: Proc Alltech's 9th Annu Symp. Nottingham University Press, Nottingham, pp 71–89
NRC (1996) Energy. In: Nutrient requirements of beef cattle, 7th rev edn. National Academy Press, Washington, DC, pp 3–15
FDA (2003) Code of Federal Regulations: Title 21, Food and Drugs. Available via USGPO. http://www.access.gpo.gov/cgi-bin/cfrassemble.cgi?title=200321. Accessed 12 Nov 2009
Weiss WP (2003) Selenium nutrition of dairy cows: comparing responses to organic and inorganic selenium forms. In: Lyons TP, Jacques KA (eds) Nutritional biotechnology in the feed and food industries: Proc Alltech's 19th Annu Symp. Nottingham University Press, Nottingham, pp 333–343
FDA (2007) Food additives permitted in feed and drinking water of animals; Selenium yeast (FR Doc. E7-13954). Fed Regist 72:39560–39562
Juniper DT, Phipps RH, Ramos-Morales E et al (2008) Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J Anim Sci 86:3100–3109
Surai PF (2006) Selenium in ruminant nutrition. In: Surai PF (ed) Selenium in nutrition and health. Nottingham University Press, Nottingham, pp 487–587
Ortman K, Pehrson B (1999) Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J Anim Sci 77:3365–3370
Peters JC, Mahan DC (2008) Effects of dietary organic and inorganic trace mineral levels on sow reproductive performances and daily mineral intakes over six parities. J Anim Sci 86:2247–2260
Bierla K, Dernovics M, Vacchina V et al (2008) Determination of selenocysteine and selenomethionine in edible animal tissues by 2D size-exclusion reversed-phase HPLC-ICP MS following carbamidomethylation and proteolytic extraction. Anal Bioanal Chem 390:1789–1798
Surai PF (2006) Molecular mechanisms of Se action: selenoproteins. In: Surai PF (ed) Selenium in nutrition and health. Nottingham University Press, Nottingham, pp 47–49
Ceballos A, Sánchez J, Stryhn H et al (2009) Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J Dairy Sci 92:324–342
Pehrson B, Knutsson M, Gyllensward M (1989) Glutathione peroxidase activity in heifers fed diets supplemented with organic and inorganic selenium compounds. Swed J Agric Res 19:53–56
Nicholson JWG, McQueen RE, Bush RS (1991) Response of growing cattle to supplementation with organically bound or inorganic sources of selenium or yeast cultures. Can J Anim 71:803–811
Gunter SA, Beck PA, Phillips JK (2003) Effects of supplementary selenium source on the performance and blood measurements in beef cows and their calves. J Anim Sci 81:856–864
Juniper DT, Phipps RH, Givens DI et al (2008) Tolerance of ruminant animals to high dose in-feed administration of a selenium-enriched yeast. J Anim Sci 86:197–204
Guyot et al (2006) Comparative responses to sodium selenite and Sel-Plex® organic selenium supplements in Belgian Blue cows and calves. In: Lyons TP, Jacques KA, Hower JM (eds) Nutritional Biotechnology in the Feed and Food Industries: Proc Alltech’s 22nd Annu Symp. Nottingham University Press, Nottingham, pp 331–340
Davis PA, McDowell LR, Van Alstyne R et al (2008) Effects of form of parenteral or dietary selenium supplementation on body weight and blood, liver, and milk concentrations in beef cows. Prof Anim Sci 24:52–59
Clyburn BS, Richardson CR, Montgomery JL et al (2001) Effect of selenium source and vitamin E level on performance and meat quality of feedlot steers. In: Lyons TP, Jacques KA (eds) Science and technology in the feed industry: Proc. Alltech's 17th Annu Symp. Nottingham University Press, Nottingham, pp 377–392
Schrauzer GN, Surai PF (2009) Selenium in human and animal nutrition: resolved and unresolved issues. A partly historical treatise in commemoration of the fiftieth anniversary of the discovery of the biological essentiality of selenium, dedicated to the memory of Klaus Schwarz (1914–1978) on the occasion of the thirtieth anniversary of his death. Crit Rev Biotechnol 29:2–9
Erwin ES, Dyer IA, Meyer TO et al (1956) Uses of aspiration biopsy technique. J Anim Sci 15:428–434
AOAC (2000) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Arlington
NRC (1996) Energy. In: Nutrient requirements of beef cattle, 7th rev. ed. National Academy Press, Washington, DC, pp 3–15
NRC (2001) Energy. In: Nutrient requirements of dairy cattle, 7th rev. ed. National Academy Press, Washington, DC, pp 13–27
Mahan DC, Brendemuhl JH, Carter SD et al (2005) Comparison of dietary selenium fed to grower-finisher pigs from various regions of the United States on resulting tissue Se and loin mineral concentrations. J Anim Sci 83:852–857
Irizarry RA, Hobbs B, Collin F et al (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Wu Z, Irizarry RA, Gentleman R et al (2004) A model based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc 99:909–917
Partek D (2009) Partek documentation: turning data into discovery. Partek Incorporated, St. Louis
Howell JA, Matthews AD, Welbourne TC et al (2003) Content of ileal EAAC1 and hepatic GLT-1 high-affinity glutamate transporters is increased in growing versus non-growing lambs, paralleling increased tissue concentrations of D- and L-glutamate and plasma glutamine and alanine. J Anim Sci 81:1030–1039
Richards CJ, Loveday HD (2004) Redefining selenium nutrition using organic selenium (Sel-Plex®): defining maximal acceptable tissue residues in beef. In: Lyons TP, Jacques KA (eds) Nutritional biotechnology in the feed and food industries: Proc. Alltech's 20th Annu Symp. Nottingham University Press, Nottingham, pp 211–220
Rao L, Puschner B, Prolla TA (2001) Gene expression profiling of low selenium status in the mouse intestine: transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress. J Nutr 131:3175–3181
Dawson KA (2006) Nutrigenomics: feeding the genes for improved fertility. Anim Reprod Sci 96:312–322
Laviola L, Natalicchio A, Giorgino F (2007) The IGF-I signaling pathway. Curr Pharm Des 13:663–669
Menuelle P, Binoux M, Plas C (1995) Regulation by insulin-like growth factor (IGF) binding proteins of IGF-II-stimulated glycogenesis in cultured fetal rat hepatocytes. Endocrinology 136:5305–5310
Laursen T (2004) Clinical pharmacological aspects of growth hormone administration. Growth Horm IGF Res 14:16–44
Ning YA, Schuller GP, Bradshaw S et al (2006) Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol Endocrinol 20:2173–2186
MacDonald RS (2000) The role of zinc in growth and cell proliferation. J Nutr 130:1500S–1508S
Foulstone EJ, Huser C, Crown AL, Holly JMP et al (2004) Differential signalling mechanisms predisposing primary human skeletal muscle cells to altered proliferation and differentiation: roles of IGF-I and TNFα. Exp Cell Res 294:223–235
Tanaka H, Moriwake T, Matsuoka Y et al (1998) Potential role of rhIGF-I/IGFBP-3 in maintaining skeletal mass in space. Bone 22(5 Suppl):145S–147S
Tilley RE, McNeil CJ, Ashworth CJ et al (2007) Altered muscle development and expression of the insulin-like growth factor system in growth retarded fetal pigs. Domest Anim Endocrinol 32:167–177
Baruch Y (2000) The liver: a large endocrine gland. J Hepatol 32:505–507
Baxter RC (2000) Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab 278:E967–E976
Baxter RC (2001) Signalling pathways involved in antiproliferative effects of IGFBP-3: a review. J Clin Pathol: Mol Pathol 54:145–148
Yamada PM, Lee K (2009) Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol 296:C954–C976
Bradshaw SL, Han VK (1993) Hormonal regulation of astroglial insulin-like growth factor (IGF)-binding protein gene expression by IGFs and insulin. Endocrinology 133:1767–1777
Smink JJ, Koster JG, Hendriks-Stegeman BI et al (1999) Insulin-like growth factor (IGF) II induced changes in expression of IGF binding proteins in lymphoid tissues of hIGF-II transgenic mice. Endocrinology 140:5876–5882
Asin-Cayuela J, Schwend T, Farge G et al (2005) The human mitochondrial transcription termination factor (mTERF) is fully active in vitro in the non-phosphorylated form. J Biol Chem 280:25499–25505
Hyvarinen AK, Pohjoismaki JLO, Reyes A et al (2007) The mitochondrial transcription termination factor mTERF modulates replication pausing in human mitochondrial DNA. Nucleic Acids Res 35:6458–6474
Yamada K, Miyamoto K (2005) Basic helix-loop-helix transcription factors, BHLHB2 and BHLHB3; their gene expressions are regulated by multiple extracellular stimuli. Front Biosci 10:3151–3171
Furukawa M, Kawamoto T, Noshiro M et al (2005) Clock gene expression in the submandibular glands. J Dent Res 84:1193–1197
Jiang X, Tian F, Du Y et al (2008) BHLHB2 controls Bdnf promoter 4 activity and neuronal excitability. J Neurosci 28:1118–1130
Fujimoto K, Hamaguchi H, Hashiba T et al (2007) Transcriptional repression by the basic helix-loop-helix protein Dec2: multiple mechanisms through E-box elements. Int J Mol Med 19:925–932
Subramaniam M, Hawse JR, Johnsen SA et al (2007) Role of TIEG1 in biological processes and disease states. J Cell Biochem 102:539–548
Subramaniam M, Hefferan TE, Tau K et al (1998) Tissue, cell type, and breast cancer stage-specific expression of a TGF-β inducible early transcription factor gene. J Cell Biochem 68:226–236
Johnsen SA, Subramaniam M, Monroe DG et al (2002) Modulation of transforming growth factor β (TGFβ)/Smad transcriptional responses through targeted degradation of TGFβ-inducible early gene-1 by human seven in absentia homologue. J Biol Chem 277:30754–30759
Pearson R, Fleetwood J, Eaton S et al (2008) Krüppel-like transcription factors: a functional family. Int J Biochem Cell Biol 40:1996–2001
Kishore U, Reid KB (2001) Structures and functions of mammalian collectins. Results Probl Cell Differ 33:225–248
Holmskov UL (2000) Collectins and collectin receptors in innate immunity. APMIS Suppl 100:1–59
Dec M, Wernicki A (2006) Conglutinin, CL-43 and CL-46—three bovine collectins. Pol J Vet Sci 9:265–275
Gérard-Monnier D, Chaudiere J (1996) Metabolism and antioxidant function of glutathione [in French]. Pathol Biol (Paris) 44:77–85
Franklin CC, Backos DS, Mohar I et al (2009) Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 30:86–98
Liu RM, Gao L, Choi J et al (1998) γ-Glutamylcysteine synthetase: mRNA stabilization and independent subunit transcription by 4-hydroxy-2-nonel. Am J Physiol 275:L861–L869
Nieto N, Mari M (2003) Cytochrome P450 2E1 responsiveness in the promoter of glutamate-cysteine ligase catalytic subunit. Hepatology 37:96–106
Suzuki A, Akimoto K, Ohno S (2003) Protein kinase Cλ/ι (PKCλ/ιa PKC isotype essential for the development of multicellular organisms. J Biol Chem 133:9–16
Fields A, Regala R (2007) Protein kinase Ci: human oncogene, prognostic marker and therapeutic target. Pharmacol Res 55:487–497
Du GS, Wang JM, Lu JX et al (2009) Expression of P-aPKC-iota, E-cadherin, and beta-catenin related to invasion and metastasis in hepatocellular carcinoma. Ann Surg Oncol 16:1578–1586
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the University of Kentucky-Alltech Nutrigenomics Alliance (JCM), NIH (NCRR-P20 RR16481 and NIEHS-2P42 ES007380-12; AJS), University of Kentucky, and Kentucky Agricultural Experiment Station (publication no. 10-07-027).
Rights and permissions
About this article
Cite this article
Liao, S.F., Brown, K.R., Stromberg, A.J. et al. Dietary Supplementation of Selenium in Inorganic and Organic Forms Differentially and Commonly Alters Blood and Liver Selenium Concentrations and Liver Gene Expression Profiles of Growing Beef Heifers. Biol Trace Elem Res 140, 151–169 (2011). https://doi.org/10.1007/s12011-010-8685-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8685-2