Abstract
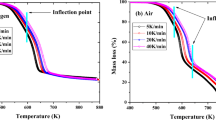
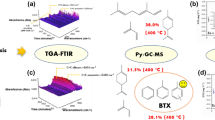
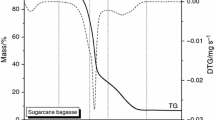
The kinetics, thermodynamics, and volatile products of camphorwood pyrolysis were investigated via thermogravimetry coupled with Fourier transform infrared (FTIR) spectroscopy at multiple heating rates. The kinetic triplets and thermodynamic parameters were estimated via model free combined with the model-fitting approach. The results showed that the pyrolysis of camphorwood in the conversion rate range from 0 to 0.85 might be considered as one-step process. The mean value of the activation energy and pre-exponential factor was 192.63 kJ/mol and 2.38 × 1013 s−1, respectively. The pyrolysis process (0 ≤ α ≤ 0.85) can be described by the three-dimensional diffusion model g(α) = [(1−α)−1/3 – 1]2. Furthermore, the predicted curves of the conversion rate α showed good agreement with the experimental curves. The values of ΔH, ΔG, and ΔS varied little with α and remained positive. In addition, the major gas products released from the camphorwood waste pyrolysis were H2O, methane, CO2, CO, C=O, O–H, C–O–C, and NH3, whose concentration in the order from highest to lowest was C=O > CO2 > O–H > H2O > methane > NH3 > C–O–C > CO. The main conclusions in the present study can provide guidance for the design and optimization of industrial reactor and selection of target biofuels or chemical raw materials.








Similar content being viewed by others
References
Sundaram, V., Muthukumarappan, K., & Gent, S. (2017). Understanding the impacts of AFEX™ pretreatment and densification on the fast pyrolysis of corn stover, prairie cord grass, and switchgrass. Applied Biochemistry and Biotechnology, 181(3), 1060–1079.
Hajer, M. A., & Pelzer, P. (2018). 2050—An energetic odyssey: Understanding ‘techniques of futuring’ in the transition towards renewable energy. Energy Research and Social Science, 44, 222–231.
Al-Hamamre, Z., Saidan, M., Hararah, M., Rawajfeh, K., Alkhasawneh, H. E., & Al-Shannag, M. (2017). Wastes and biomass materials as sustainable-renewable energy resources for Jordan. Renewable and Sustainable Energy Reviews, 67, 295–314.
Singer, S., Denruyter, J. P., & Yener, D. (2017). The energy report: 100% renewable energy by 2050. Tata Energy Research Institute India, 379–383.
Toscano, G., Duca, D., Rossini, G., Mengarelli, C., & Pizzi, A. (2015). Identification of different woody biomass for energy purpose by means of soft independent modeling of class analogy applied to thermogravimetric analysis. Energy, 83, 351–357.
Johansson, L. S., Tullin, C., Leckner, B., & Sjövalla, P. (2003). Particle emissions from biomass combustion in small combustors. Biomass and Bioenergy, 25(4), 435–446.
Zhang, X., Chen, Q., Bradford, R., Sharifi, V., & Swithenbank, J. (2010). Experimental investigation and mathematical modelling of wood combustion in a moving grate boiler. Fuel Processing Technology, 91(11), 1491–1499.
Mensah, R. A., Jiang, L., Asante-Okyere, S., Xu, Q., & Jin, C. (2019). Comparative evaluation of the predictability of neural network methods on the flammability characteristics of extruded polystyrene from microscale combustion calorimetry. Journal of Thermal Analysis and Calorimetry, 138(5), 3055–3064.
Kalinoski, R. M., Flores, H. D., Thapa, S., & Tuegel, E. (2017). Pretreatment of hardwood and miscanthus with trametes versicolor for bioenergy conversion and densification strategies. Applied Biochemistry and Biotechnology, 183(4), 1401–1413.
Samavi, M., Uprety, B. K., & Rakshit, S. (2019). Bioconversion of poplar wood hemicellulose prehydrolysate to microbial oil using cryptococcus curvatus. Applied Biochemistry and Biotechnology, 189(6), 626–637.
Sitepu, I. R., Garay, L. A., Sestric, R., Levin, D., Block, D. E., German, J. B., & Boundy-Mills, K. L. (2014). Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnology Advances, 32(7), 1336–1360.
Ding, Y., Zhang, W., Yu, L., & Lu, K. (2019). The accuracy and efficiency of GA and PSO optimization schemes on estimating reaction kinetic parameters of biomass pyrolysis. Energy, 176, 582–588.
Ding, Y., Ezekoye, O. A., Zhang, J., Wang, C., & Lu, S. (2018). The effect of chemical reaction kinetic parameters on the bench-scale pyrolysis of lignocellulosic biomass. Fuel, 232, 147–153.
Fei, Y., Deng, S., Chen, P., Liu, Y., Wan, Y., Olson, A., et al. (2007). Physical and chemical properties of bio-oils from microwave pyrolysis of corn stover. Applied Biochemistry and Biotechnology, 137(1–12), 957–970.
Ding, Y., Wang, C., Chaos, M., Chen, R., & Lu, S. (2016). Estimation of beech pyrolysis kinetic parameters by shuffled complex evolution., 200, 658–665.
Ding, Y., Zhang, J., He, Q., Huang, B., & Mao, S. (2019). The application and validity of various reaction kinetic models on woody biomass pyrolysis. Energy, 179, 784–791.
Ding, Y., EO, A., Jiaqing, Z., Changjian, W., & Shouxiang, L. (2018). The effect of chemical reaction kinetic parameters on the bench-scale pyrolysis of lignocellulosic biomass. Fuel, 232, 147–153.
Ding, Y., Fukumoto, K., Ezekoye, O. A., Lu, S., Wang, C., & Li, C. (2020). Experimental and numerical simulation of multi-component combustion of typical charring material. Combustion and Flame, 211, 417–429.
Ding, Y., Huang, B., Li, K., Du, W., Lu, K., & Zhang, Y. (2020). Thermal interaction analysis of isolated hemicellulose and cellulose by kinetic parameters during biomass pyrolysis. Energy, 117010.
Kumar, G., Dharmaraja, J., Arvindnarayan, S., Shoban, S., Bakonyi, P., Saratale, G. D., et al. (2019). A comprehensive review on thermochemical, biological, biochemical and hybrid conversion methods of bio-derived lignocellulosic molecules into renewable fuels. Fuel, 251, 352–367.
Gao, X., Jiang, L., & Xu, Q. (2020). Experimental and theoretical study on thermal kinetics and reactive mechanism of nitrocellulose pyrolysis by traditional multi kinetics and modeling reconstruction. Journal of Hazardous Materials, 386, 121645.
Zoltán, T., Tamás, V., János, S., Norbert, M., & Tibor, C. (2018). Kinetic identification of plastic waste pyrolysis on zeolite-based catalysts. Energy Conversion and Management, 173, 320–330.
Wei, Z., Zeng, G., Kosa, M., Huang, D., & Ragauskas, A. J. (2015). Pyrolysis oil-based lipid production as biodiesel feedstock by rhodococcus opacus. Applied Biochemistry and Biotechnology, 175(2), 1234–1246.
Hassan, E. B. M., Steele, P. H., & Ingram, L. (2009). Characterization of fast pyrolysis bio-oils produced from pretreated pine wood. Applied Biochemistry and Biotechnology, 154(1–3), 3–13.
Feng-Wen, Y., Deng-Xiang, J., Yong, N., Yao, L., Cheng-Jie, H., & Jian-Bing, J. (2012). Study on the pyrolysis of cellulose for bio-oil with mesoporous molecular sieve catalysts. Applied Biochemistry and Biotechnology, 168(1), 174–182.
Mishra, G., Kumar, J., & Bhaskar, T. (2015). Kinetic studies on the pyrolysis of pinewood. Bioresource Technology, 182, 282–288.
Ding, Y., Ezekoye, O. A., Lu, S., Wang, C., & Zhou, R. (2017). Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. Energy Conversion and Management, 132, 102–109.
Maia, A. A., & de Morais, L. C. (2016). Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresource Technology, 204, 157–163.
Bach, Q. V., Trinh, T. N., Tran, K. Q., & Thi, N. B. D. (2017). Pyrolysis characteristics and kinetics of biomass torrefied in various atmospheres. Energy Conversion and Management, 141, 72–78.
Chen, Z., Hu, M., Zhu, X., Guo, D., Liu, S., Hu, Z., Xiao, B., Wang, J., & Laghari, M. (2015). Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresource Technology, 192, 441–450.
Ghadikolaei, S. S., Omrani, A., & Ehsani, M. (2017). Non-isothermal degradation kinetics of ethylene-vinyl acetate copolymer nanocomposite reinforced with modified bacterial cellulose nanofibers using advanced isoconversional and master plot analyses. Thermochimica Acta, 655, 87–93.
Mishra, R. K., & Mohanty, K. (2018). Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresource Technology, 251, 63–74.
Fanfan, X., Bo, W., Dan, Y., Junhui, H., Yingyun, Q., & Yuanyu, T. (2018). Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Conversion and Management.
Ding, Y., Ezekoye, O. A., Lu, S., & Wang, C. (2016). Thermal degradation of beech wood with thermogravimetry/Fourier transform infrared analysis. Energy Conversion and Management, 120, 370–377.
Liang, F., Wang, R., Xiang, H., Yang, X., & Zhang, T. (2018). Investigating pyrolysis characteristics of moso bamboo through TG-FTIR and Py-GC/MS. Bioresource Technology, 256, 53–60.
Cai, H., Zou, H., Liu, J., Xie, W., Kuo, J., Buyukada, M., & Evrendilek, F. (2018). Thermal degradations and processes of waste tea and tea leaves via TG-FTIR: Combustion performances, kinetics, thermodynamics, products and optimization. Bioresource Technology, 268, 715–725.
Jiang, H., Wang, J., Wu, S., Wang, B., & Wang, Z. (2010). Pyrolysis kinetics of phenol–formaldehyde resin by non-isothermal thermogravimetry. Carbon, 48(2), 352–358.
Vlaev, L., Nedelchev, N., Gyurova, K., & Zagorcheva, M. (2008). A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. Journal of Analytical and Applied Pyrolysis, 81(2), 253–262.
Liqing, L., & Donghua, C. (2004). Application of iso-temperature method of multiple rate to kinetic analysis. Journal of Thermal Analysis and Calorimetry, 78(1), 283–293.
Kissinger, H. (1956). Variation of peak temperature with heating rate in differential thermal analysis. Journal of Research of the National Bureau of Standards, 57, 217–221.
Akahira, T., & Sunose, T. (1971). Method of determining activation deterioration constant of electrical insulating materials. Science and Technology, 16, 22–31.
Flynn, J. H., & Wall, L. A. (1966). A quick, direct method for the determination of activation energy from thermogravimetric data. Journal of Polymer Science Part C Polymer Letters, 4(5), 323–328.
Takeo, O. (1965). A new method of analyzing thermogravimetric data. Bulletin of the Chemical Society of Japan, 38, 1881–1886.
Coats, A. W., & Redfern, J. P. (1964). Kinetic parameters from thermogravimetric data. Nature, 201(4914), 68–69.
Chen, J., Liu, J., He, Y., Huang, L., Sun, S., Sun, J., Chang, K., Kuo, J., Huang, S., & Ning, X. (2017). Investigation of co-combustion characteristics of sewage sludge and coffee grounds mixtures using thermogravimetric analysis coupled to artificial neural networks modeling. Bioresource Technology, 225, 234–245.
Tian, L., Shen, B., Xu, H., Li, F., Wang, Y., & Singh, S. (2016). Thermal behavior of waste tea pyrolysis by TG-FTIR analysis. Energy, 103, 533–542.
Vyazovkin, S. (2000). Computational aspects of kinetic analysis.: Part C. The ICTAC kinetics project — The light at the end of the tunnel? Thermochimica Acta, 355(1), 155–163.
Ahmad, M. S., Mehmood, M. A., Liu, C. G., Tawab, A., Bai, F. W., Sakdaronnarong, C., et al. (2018). Bioenergy potential of Wolffia arrhiza appraised through pyrolysis, kinetics, thermodynamics parameters and TG-FTIR-MS study of the evolved gases. Bioresource Technology, 253.
Vlaev, L. T., Georgieva, V. G., & Genieva, S. D. (2007). Products and kinetics of non-isothermal decomposition of vanadium(IV) oxide compounds. Journal of Thermal Analysis and Calorimetry, 88(3), 805–812.
Zhang, J., Liu, J. Y., Evrendilek, F., Xie, W., & Buyukada, M. (2019). Kinetics, thermodynamics, gas evolution and empirical optimization of cattle manure combustion in air and oxy-fuel atmospheres. Applied Thermal Engineering, 149, 119–131.
Xu, Y., & Chen, B. (2013). Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresource Technology, 146(10), 485–493.
Wang, S., Tang, Y., Schobert, H. H., Guo, Y. N., Gao, W., & Lu, X. (2013). FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China. Journal of Analytical and Applied Pyrolysis, 100(6), 75–80.
Liu, Q., Wang, S., Zheng, Y., Luo, Z., & Cen, K. (2008). Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. Journal of Analytical and Applied Pyrolysis, 82(1), 170–177.
Gao, N., Li, A., Cui, Q., Lin, D., & Yue, D. (2013). TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. Journal of Analytical and Applied Pyrolysis, 100(6), 26–32.
Yang, H., Yan, R., Chen, H., Lee, D. H., & Zheng, C. (2007). Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel, 86(12–13), 1781–1788.
Yang, J., Chen, H., Zhao, W., & Zhou, J. (2016). TG–FTIR-MS study of pyrolysis products evolving from peat. Journal of Analytical and Applied Pyrolysis, 117, 296–309.
Zhi, Z., Changjian, W., Gai, H., Haoran, L., Shenlin, Y., & Aifeng, Z. (2018). Thermal degradation behaviors and reaction mechanism of carbon fibre-epoxy composite from hydrogen tank by TG-FTIR. Journal of Hazardous Materials, 357, 73–80.
Granada, E., Eguía, P., Vilan, J. A., Comesaña, J. A., & Comesaña, R. (2012). FTIR quantitative analysis technique for gases. Application in a biomass thermochemical process. Renewable Energy, 41(none), 416–421.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51806106) and the Science and Technology Department of Jiangsu Province, China (No: BK20170838).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, X., Pan, R., Li, P. et al. Kinetics, Thermodynamics, and Volatile Products of Camphorwood Pyrolysis in Inert Atmosphere. Appl Biochem Biotechnol 191, 1605–1623 (2020). https://doi.org/10.1007/s12010-020-03300-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03300-2