Abstract
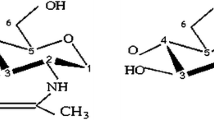
Solid wastes generated from the seafood industry represent an important environmental pollutant; therefore, utilization of those wastes for the development of processing biochemical tools could be an attractive and clean solution for the seafood industry. This study reports the immobilization of semi-purified acidic proteases from Monterey sardine stomachs onto chitin and chitosan materials extracted from shrimp head waste. Several supports (chitosan beads, chitosan flakes, and partially deacetylated flakes) were activated either with genipin or Na-tripolyphosphate and evaluated as a mean to immobilize acidic proteases. The protein load varied within the 67–91 % range on different supports. The immobilization systems based on chitosan beads achieved the highest protein loads but showed the lowest retained catalytic activities. The best catalytic behavior was obtained using partially deacetylated chitin flakes activated either with genipin or Na-tripolyphosphate. According to results, the immobilization matrix structure, as well as acetylation degree of chitin–chitosan used, has considerable influence on the catalytic behavior of immobilized proteases. Partially deacetylated chitin flakes represent a suitable option as support for enzyme immobilization because its preparation requires fewer steps than other supports. Two abundant seafood by-products were used to obtain a catalytic system with enough proteolytic activity to be considered for biotechnological applications in diverse fields.




Similar content being viewed by others
References
Brady, D., & Jordaan, J. (2009). Biotechnology Letter, 31, 1639–1650.
Altun, G. D., & Cetinus, S. A. (2007). Food Chemistry, 100, 964–971.
Phanturat, P., Benjakul, S., Visessanguan, W., & Roytrakul, S. (2010). LWT- Food Science and Technology, 43, 86–97.
Khaled, H. B., Ghorbel-Bellaaj, O., Hmidet, N., Jellouli, K., Ali, N. E. H., Ghorbel, S., et al. (2011). Food Chemistry, 128, 847–853.
Castillo-Yañez, F. J., Pacheco-Aguilar, R., Garcia-Carreño, F. L., & Del-Toro, M. A. (2004). Food Chemistry, 85, 343–350.
Singh, A. N., Suthar, N., SINGH, S., & Dubey, V. K. (2011). Journal Agriculture Food and Chemistry, 59, 6256–6262.
Kılınç, D. A., Teke, M., Önal, S., & Telefoncu, A. (2006). Preparative Biochemistry and Biotechnology, 36, 153–163.
Krajewska, B. (2004). Enzyme and Microbial Technology, 35, 126–139.
Chiou, S. H., Hung, T. C., Giridhar, R., & Wu, W. T. (2007). Preparative Biochemistry and Biotechnology, 37, 265–275.
Wang, W., Jiang, Y., Zhou, L., & Gao, J. (2011). Applied Biochemistry and Biotechnology, 164, 561–572.
Sangeetha, K., & Emilia, A. T. (2008). Journal of Applied Polymer Science, 107, 2899–2908.
He, S., Franco, C., & Zhang, W. (2013). Food Research International, 50, 289–297.
Morrissey, M. (2001) Book of abstracts, 2001 IFT Annual Meeting. New Orleans, USA
Arvanitoyannis, I. S., & Kassaveti, A. (2007). International Journal of Food Science and Technology, 43, 726–745.
Dıaz-Lopez, M., Moyano-Lopez, F. J., Alarcon-Lopez, F. J., Garcia-Carreño, F. L., & NavarretedelToro, M. (1998). Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology, 121, 369–377.
Stauffer, C. (1989). Enzyme assays for food scientists. New York: Van Nostrand Reinhold.
García Carreño, F. L., & Haard, N. F. (1993). Journal of Food Biochemistry, 17, 97–113.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Beaney, P., Lizardi Mendoza, J., & Healy, M. (2005). Journal of Chemical Technology and Biotechnology, 80, 145–150.
Brugnerotto, J., Lizardi, J., Goycoolea, F., Argüelles-Monal, W., Desbrieres, J., & Rinaudo, M. (2001). Polymer, 42, 3569–3580.
Bradford, M. (1976). Analytical Biochemistry, 72, 248–25.
Novick, S. J., & Rozzell, J. D. (2005). Immobilization of enzymes by covalent attachment. In J. L. Barredo (Ed.), Microbial enzymes and biotransformations (pp. 247–272). Totowa: Humana.
Magnin, D., Dumitriu, S., & Chornet, E. (2003). Journal of Bioactive and Compatible Polymers, 18, 355–373.
Gildberg, A. (1988). Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology, 91, 425–435.
Villalba-Villalba, A. G., Pacheco-Aguilar, R., Ramirez-Suarez, J. C., Valenzuela-Soto, E. M., Castillo-Yáñez, F. J., & Márquez-Ríos, E. (2011). Fisheries Science, 77, 1–9.
Benkhelifa, H., Bengoa, C., Larre, C., Guibal, E., Popineau, Y., & Legrand, J. (2005). Process Biochemistry, 40, 461–467.
Kilinc, A., Onal, S., & Telefoncu, A. (2002). Turkish Journal of Chemistry, 26, 311–316.
Jiang, D. S., Long, S. Y., Huang, J., Xiao, H. Y., & Zhou, J. Y. (2005). Biochemical Engineering Journal, 25, 15–23.
Dhananjay, S., & Mulimani, V. (2008). Journal of Food Biochemistry, 32, 521–535.
Kasaai, M. R. (2010). Carbohydrate Polymers, 79, 801–810.
Acknowledgments
The authors wish to thank Karla Martinez Robinson for her technical assistance. Jesus Aaron Salazar-Leyva was on a graduate leave supported by both the Universidad Politecnica de Sinaloa and the Consejo Nacional de Ciencia y Tecnologia of Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salazar-Leyva, J.A., Lizardi-Mendoza, J., Ramirez-Suarez, J.C. et al. Acidic Proteases from Monterey Sardine (Sardinops sagax caerulea) Immobilized on Shrimp Waste Chitin and Chitosan Supports: Searching for a By-product Catalytic System. Appl Biochem Biotechnol 171, 795–805 (2013). https://doi.org/10.1007/s12010-013-0407-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0407-8