Abstract
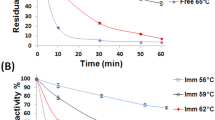
Lactose has been hydrolyzed using covalently immobilized β-galactosidase on thermally stable carrageenan coated with chitosan (hydrogel). The hydrogel’s mode of interaction was proven by Fourier transform infrared spectroscopy, differential scanning calorimetry (DSC), and Schiff’s base formation. The DSC thermogram proved the formation of a strong polyelectrolyte complex between carrageenan and chitosan followed by glutaraldehyde as they formed one single peak. The modification of carrageenan improved the gel’s thermal stability in solutions from 35 °C to 95 °C. The hydrogel has been proven to be efficient for β-galactosidase immobilization where 11 U/g wet gel was immobilized with 50% enzyme loading capacity. Activity and stability of free and immobilized β-galactosidase towards pH and temperature showed marked shifts in their optimum pH from 4.5–5 to 5–5.5 and temperature from 50 °C to 45–55 °C after immobilization, which reveals higher catalytic activity and reasonable stability at wider pHs and temperatures. The apparent K m of the immobilized enzyme increased from 13.2 to 125 mM, whereas the V max increased from 3.2 to 6.6 μmol/min compared to the free enzyme, respectively. The free and immobilized enzymes showed lactose conversion of 87% and 70% at 7 h, respectively. The operational stability showed 97% retention of the enzyme activity after 15 uses, which demonstrates that the covalently immobilized enzyme is unlikely to leach. The new carrier could be suitable for immobilization of other industrial enzymes.








Similar content being viewed by others
References
Ordoñez, J. A., Cambero, M. A., Fernandez, L., Garcia, M. L., Garcia, G., Hoz, L. et al. (1998). Tecnologia de los alimentos (II). Madrid, Spain: Editorial Sintesis.
Richmond, M. (1759). Gray, J. & Stine, C. (1981). Journal of dairy science, 1759, 64.
German, J. H. (1997). Applied enzymology of lactose hydrolysis. In Milk powders for the future, p. 81.
Sungur, S., & Akbulut, U. (1994). Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 59, 303. doi:10.1002/jctb.280590314.
Nijipels, H. H. (1981). Lactases and their applications. In G. G. Birch, H. Blakebrough, & K. J. Parker (Eds.), Enzyme and food processing (p. 89). London: Applied Science Publishers.
Betancor, L., Luckarift, R., Seo, H., Brand, O., & Spain. (2008). Biotechnology and Bioengineering, 99, 261. doi:10.1002/bit.21570.
Wang, Y., Xu, J., Luo, G., & Dai, Y. (2008). Bioresource Technology, 99, 2299. doi:10.1016/j.biortech.2007.05.014.
Jozef, S., & Sylwia, W. (2006). Enzyme and Microbial Technology, 39, 1417. doi:10.1016/j.enzmictec.2006.03.028.
Salah, S., Srimathi, S., Gulnara, S., Ikuo, S., & Bengt, D. (2008). Talanta, 77, 490. doi:10.1016/j.talanta.2008.04.003.
D’Souza, S. F. (1999). Current Science, 77, 69.
Kahraman, V., Bayramoglu, G., Kayaman-Apohan, N., & Atilla, G. (2007). Food Chemistry, 104, 1385. doi:10.1016/j.foodchem.2007.01.054.
Shweta, P., Shamsher, K., Ghanshyam, C., & Reena, G. (2008). Bioresource Technology, 99, 2566. doi:10.1016/j.biortech.2007.04.042.
Emese, B., Ágnes, N., Csaba, S., Tivadar, F., & János, G. (2008). Journal of Biochemical and Biophysical Methods, 70, 1240. doi:10.1016/j.jprot.2007.11.005.
Peppler, H. J., & Reed, G. (1987). Enzymes in food and feed processing. In H. J. Rehm, G. Reed (Eds.), Biotechnology, vol. 7a (p. 578). Weinheim: VCH.
Elnashar, M. M., & Yassin, A. M. (2008). Journal of Applied Polymer Science (in press).
Wang, J., & Qiang, Y. (1999). Chemosphere, 38, 3109. doi:10.1016/S0045-6535(98)00516-5.
Elnashar, M. M., Millner, P., Johnson, A., & Gibson, T. (2005). Biotechnology Letters, 27, 737. doi:10.1007/s10529-005-5363-0.
Luong, j. (1985). Biotechnology and Bioengineering, 27, 1652.
Chang, J., Cho, C., & Chen, S. (2001). Process Biochemistry, 36, 757. doi:10.1016/S0032-9592(00)00274-0.
Moon, S., & Parulekar, S. J. (1991). Biotechnology Progress, 7, 516. doi:10.1021/bp00012a006.
Tramper, J., & Grootjen, D. R. (1986). Enzyme and Microbial Technology, 8, 477. doi:10.1016/0141-0229(86)90051-7.
Chao, K. C., Haugen, M. M., & Royer, G. P. (1986). Biotechnology and Bioengineering, 28, 1289. doi:10.1002/bit.260280902.
Trinder, P. (1969). Annals of Clinical Biochemistry, 6, 24.
El-Masry, M., De Maioa, B., Martelli, C., Casadio, C., Moustafa, A., Rossi, A., et al. (2001). Journal of Molecular Catalysis. B, Enzymatic, 16, 175. doi:10.1016/S1381-1177(01)00061-3.
El-Masry, M., Elnashar, M. M., & El-sherif, M. (2007). Journal of Applied Polymer Science, 106, 3571. doi:10.1002/app.26931.
Elnashar, M. M., Yassin, A. M., & Kahil, T. (2008). Journal of Applied Polymer Science, 109, 4105. doi:10.1002/app.28379.
Tapia, C., Escobara, Z., Costab, E., Sapag-Hagara, J., Valenzuelaa, F., Basualtoa, C., et al. (2004). European Journal of Pharmaceutics and Biopharmaceutics, 57, 65. doi:10.1016/S0939-6411(03)00153-X.
Maciel, J. S., Silva, D. A., Haroldo, C. B., & Paula, R. C. (2005). European Polymer Journal, 41, 2726. doi:10.1016/j.eurpolymj.2005.05.009.
Desai, P., Dave, A., & Devi, S. (2004). Journal of Molecular Catalysis. B, Enzymatic, 31, 143. doi:10.1016/j.molcatb.2004.08.004.
Tor, R., Dror, Y., & Freeman, A. (1989). Enzyme and Microbial Technology, 11, 306. doi:10.1016/0141-0229(89)90047-1.
Tanriseven, A., & Dogan, S. (2002). Process Biochemistry, 38, 27. doi:10.1016/S0032-9592(02)00049-3.
Szczodrakr, J. (2000). Journal of Molecular Catalysis B: Enzymatic, 10, 631. 29.
Kennedy, J., & Cabral, M. (1987). Enzyme immobilization. In J. Kennedy, J. Rehm, G. Reed (Eds.), Biotechnology, Vol. 7a, Chapter 7. Germany: VCH.
Mohi Eldin, M., Rossi, S., Bencivenga, U., & Mita, J. (1999). Molecular Catalysis B. Enzymatic, 47, 1.
Haider, T., & Husain, Q. (2007). Journal of the Science of Food and Agriculture, 87, 1278. doi:10.1002/jsfa.2840.
Nakane, K., Ogihara, T., Ogata, N., & Kurokawa, Y. (2001). Journal of Applied Polymer Science, 81, 2084. doi:10.1002/app.1642.
Acknowledgment
This work was supported by the National Research Centre, Centre of Scientific Excellence, Laboratory of Advanced Materials & Nanotechnology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elnashar, M.M.M., Yassin, M.A. Lactose Hydrolysis by β-Galactosidase Covalently Immobilized to Thermally Stable Biopolymers. Appl Biochem Biotechnol 159, 426–437 (2009). https://doi.org/10.1007/s12010-008-8453-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8453-3