Abstract
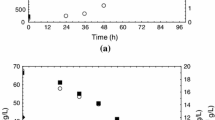
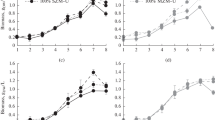
With the objective of determining the kinetic behavior (growth, substrate, pH, and carotenoid production) and obtain the stoichiometric parameters of the fermentative process by Sporidiobolus salmonicolor in synthetic and agroindustrial media, fermentations were carried out in shaken flasks at 25°C, 180 rpm, and initial pH of 4.0 for 120 h in the dark, sampling every 6 h. The maximum concentrations of total carotenoids in synthetic (913 μg/L) and agroindustrial (502 μg/L) media were attained approximately 100 h after the start of the fermentative process. Carotenoid bioproduction is associated with cell growth and the ratio between carotenoid production and cell growth (Y P/X) is 176 and 163 μg/g in the synthetic and agroindustrial media, respectively. The pH of the agroindustrial fermentation medium varied from 4.2 to 8.5 during the fermentation. The specific growth rate (μ X) for S. salmonicolor in synthetic and agroindustrial media was 0.07 and 0.04 h−1, respectively. The synthetic medium allowed for greater productivity, obtaining maximum cell productivity (P x) of 0.08 g L−1 h−1 and maximum total carotenoid productivity (P car) of 14.2 μg L−1 h−1. Knowledge of the kinetics of a fermentative process is of extreme importance when transposing a laboratory experiment to an industrial scale, as well as making a quantitative comparison between different culture conditions.







Similar content being viewed by others
References
Botella-Pavía, P., & Rodríguez-Concepción, M. (2006). Physiologia Plantarum, 126, 369–381. doi:10.1111/j.1399-3054.2006.00632.x.
Hu, Z.-C., Zheng, Y.-G., Wang, Z., & Shen, Y.-C. (2006). Enzyme and Microbial Technology, 39, 586–590. doi:10.1016/j.enzmictec.2005.11.017.
Liu, Y.-S., Wu, J.-Y., & Ho, K.-P. (2006). Biochemical Engineering Journal, 27, 331–335. doi:10.1016/j.bej.2005.08.031.
Buzzini, P., Martini, A., Gaetani, M., Turchetti, B., Pagnoni, U. M., & Davoli, P. (2005). Enzyme and Microbial Technology, 36, 687–692. doi:10.1016/j.enzmictec.2004.12.028.
Dufosse, L., Galaup, P., Yaron, A., Arad, S. M., Blanc, P., Murthy, K. N. C., et al. (2005). Trends in Food Science & Technology, 16, 389–406. doi:10.1016/j.tifs.2005.02.006.
Tinoi, J., Rakariyatham, R. L., & Deming, R. L. (2005). Process Biochemistry, 40, 2551–2557. doi:10.1016/j.procbio.2004.11.005.
Davoli, P., Mierau, V., & Weber, R. W. S. (2004). Applied Biochemistry and Microbiology, 40, 392–397. doi:10.1023/B:ABIM.0000033917.57177.f2.
Hiss, H. (2001). Cinética de processos fermentativos. In W. Schmidell, U. A. Lima, E. Aquarone, & W. Borzani (Eds.), Biotecnologia Industrial: Engenharia Bioquímica (pp. 93–121). São Paulo: Editora Edgar Blücher Ltda.
Bailey, J. E., & Ollis, D. F. (1986). In biochemical engineering fundamentals (2nd ed.). New York: McGraw-Hill.
Valduga, E., Treichel, H., Valério, A., Jacques, R., Furigo Júnior, A., & Di Luccio, M. (2007). Quimica Nova, 30, 1860–1866. doi:10.1590/S0100-40422007000800012.
Valduga, E., Treichel, H., Valério, A., Furigo Júnior, A., & Di Luccio, M. (2008). Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), in press.
Davies, B. H. (1976). In T. W. Goodwin (Ed.), Chemistry and biochemistry of plant pigments. New York: Academic.
Maldonado, I. R., Rodriguez-Amaya, D. B., & Scamparini, A. R. P. (2008). Food Chemistry, 107, 145–150. doi:10.1016/j.foodchem.2007.07.075.
Mantzouridou, F., Roukas, T., & Kotzekidou, P. (2002). Biochemical Engineering Journal, 10, 123–135. doi:10.1016/S1369-703X(01)00166-8.
Frengova, G., Simova, E., Pavlova, K., Beshkova, D. M., & Grigrova, D. (1994). Biotechnology and Bioengineering, 44, 888–894. doi:10.1002/bit.260440804.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valduga, E., Valério, A., Treichel, H. et al. Kinetic and Stoichiometric Parameters in the Production of Carotenoids by Sporidiobolus salmonicolor (CBS 2636) in Synthetic and Agroindustrial Media. Appl Biochem Biotechnol 157, 61–69 (2009). https://doi.org/10.1007/s12010-008-8383-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8383-0