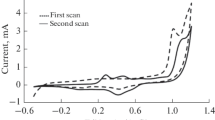
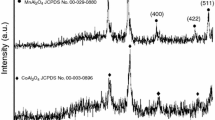
Abstract
Polypyrrole (PPy) doped with either tungstate or vanadate as counter anions was synthesized by chemical oxidative polymerization on the surface of aluminum (Al) flakes. This resulted in the deposition of PPy on the surface of the Al flakes leading to the formation of doped PPy/Al flake composite pigments. These composite pigments were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, energy dispersive spectroscopy, conductive-atomic force microscopy, four-point probe conductivity, and X-ray photoelectron spectroscopy. Furthermore, these composites were incorporated in an epoxy-amide binder system in order to formulate a primer for an aluminum 2024-T3 substrate. The coatings were exposed to the Prohesion test conditions and corrosion resistance properties were monitored by electrochemical impedance spectroscopy, DC polarization, galvanic coupling, and scanning electrochemical microscopy measurements. It was found that the doped PPy/Al flake coatings provided sacrificial protection to the underlying aluminum 2024-T3 substrate. Additionally, the release of dopants from PPy backbone resulted in the passivation in the defect areas improving the corrosion protection ability.























Similar content being viewed by others
References
Ahmad, Z, Principles of Corrosion Engineering and Corrosion Control. Elsevier Science, Oxford, 2006
Jones, DA, Principles and Prevention of Corrosion. Prentice Hall, New York, 1996
Revie, RW, Uhlig’s Corrosion Handbook. Wiley, New York, 2011
Skotheim, TA, Reynolds, JR, Conjugated Polymers: Processing and Applications. CRC Press, Boca Raton, 2007
Cohen, SM, “Review: Replacements for Chromium Pretreatments on Aluminum.” Corrosion, 51 (1) 71–78 (1995)
Cohen, MD, Kargacin, B, Klein, CB, Costa, M, “Mechanisms of Chromium Carcinogenicity and Toxicity.” Crit. Rev. Toxicol., 23 (3) 255–281 (1993)
Macdiarmid, AG, Chiang, JC, Richter, AF, Epstein, AJ, “Polyaniline: A New Concept in Conducting Polymers.” Synth. Met., 18 (1–3) 285–290 (1987)
Heinze, J, “Electronically Conducting Polymers Electrochemistry IV.” In: Steckhan, E (ed.) Topics in Current Chemistry, pp. 1–47. Springer, Berlin, 1990
Stenger-Smith, JD, “Intrinsically Electrically Conducting Polymers. Synthesis, Characterization, and Their Applications.” Prog. Polym. Sci., 23 (1) 57–79 (1998)
Inzelt, G, Conducting Polymers: A New Era in Electrochemistry. Springer, Berlin, 2012
Hosseini, MG, Raghibi-Boroujeni, M, Ahadzadeh, I, Najjar, R, Seyed Dorraji, MS, “Effect of Polypyrrole–Montmorillonite Nanocomposites Powder Addition on Corrosion Performance of Epoxy Coatings on Al 5000.” Prog. Org. Coat., 66 (3) 321–327 (2009)
Castagno, K, Azambuja, D, Dalmoro, V, “Polypyrrole Electropolymerized on Aluminum Alloy 1100 Doped with Oxalate and Tungstate Anions.” J. Appl. Electrochem., 39 (1) 93–100 (2009)
Hosseini, MG, Sabouri, M, Shahrabi, T, “Comparison of the Corrosion Protection of Mild Steel by Polypyrrole–Phosphate and Polypyrrole–Tungstenate Coatings.” J. Appl. Polym. Sci., 110 (5) 2733–2741 (2008)
Bidan, G, “Electroconducting Conjugated Polymers: New Sensitive Matrices to Build Up Chemical or Electrochemical Sensors. A Review.” Sens. Actuators B, 6 (1–3) 45–56 (1992)
Inzelt, G, Pineri, M, Schultze, JW, Vorotyntsev, MA, “Electron and Proton Conducting Polymers: Recent Developments and Prospects.” Electrochim. Acta, 45 (15–16) 2403–2421 (2000)
Otero, TF, Angulo, E, Rodríguez, J, Santamaría, C, “Electrochemomechanical Properties from a Bilayer: Polypyrrole/Non-conducting and Flexible Material—Artificial Muscle.” J. Electroanal. Chem., 341 (1–2) 369–375 (1992)
Rudge, A, Davey, J, Raistrick, I, Gottesfeld, S, Ferraris, JP, “Conducting Polymers as Active Materials in Electrochemical Capacitors.” J. Power Sources, 47 (1–2) 89–107 (1994)
Scrosati, B, “Conducting Polymers: Advanced Materials for New Design, Rechargeable Lithium Batteries.” Polym. Int., 47 (1) 50–55 (1998)
Cai, Z, Geng, M, Tang, Z, “Novel Battery Using Conducting Polymers: Polyindole and Polyaniline as Active Materials.” J. Mater. Sci., 39 (12) 4001–4003 (2004)
Braun, D, Heeger, AJ, “Electroluminescence from Light-Emitting Diodes Fabricated from Conducting Polymers.” Thin Solid Films, 216 (1) 96–98 (1992)
Dai, L, Winkler, B, Dong, L, Tong, L, Mau, AWH, “Conjugated Polymers for Light-Emitting Applications.” Adv. Mater., 13 (12–13) 915–925 (2001)
Lira, LM, Córdoba de Torresi, SI, “Conducting Polymer–Hydrogel Composites for Electrochemical Release Devices: Synthesis and Characterization of Semi-interpenetrating Polyaniline–Polyacrylamide Networks.” Electrochem. Commun., 7 (7) 717–723 (2005)
Miller, LL, “Electrochemically Controlled Release of Drug Ions from Conducting Polymers.” Mol. Cryst. Liquid Cryst., 160 (1) 297–301 (1988)
Saito, Y, Azechi, T, Kitamura, T, Hasegawa, Y, Wada, Y, Yanagida, S, “Photo-Sensitizing Ruthenium Complexes for Solid State Dye Solar Cells in Combination with Conducting Polymers as Hole Conductors.” Coord. Chem. Rev., 248 (13–14) 1469–1478 (2004)
Wang, Y, Jing, X, “Intrinsically Conducting Polymers for Electromagnetic Interference Shielding.” Polym. Adv. Technol., 16 (4) 344–351 (2005)
Lakshmi, K, John, H, Mathew, KT, Joseph, R, George, KE, “Microwave Absorption, Reflection and EMI Shielding of PU–PANI Composite.” Acta Mater., 57 (2) 371–375 (2009)
Sitaram, S, Stoffer, J, O’Keefe, T, “Application of Conducting Polymers in Corrosion Protection.” J. Coat. Technol., 69 (866) 65–69 (1997)
Tallman, D, Spinks, G, Dominis, A, Wallace, G, “Electroactive Conducting Polymers for Corrosion Control.” J. Solid State Electrochem., 6 (2) 73–84 (2002)
Spinks, G, Dominis, A, Wallace, G, Tallman, D, “Electroactive Conducting Polymers for Corrosion Control.” J. Solid State Electrochem., 6 (2) 85–100 (2002)
Ahmad, N, MacDiarmid, AG, “Inhibition of Corrosion of Steels with the Exploitation of Conducting Polymers.” Synth. Met., 78 (2) 103–110 (1996)
Breslin, CB, Fenelon, AM, Conroy, KG, “Surface Engineering: Corrosion Protection Using Conducting Polymers.” Mater. Des., 26 (3) 233–237 (2005)
Rammelt, U, Nguyen, PT, Plieth, W, “Corrosion Protection by Ultrathin Films of Conducting Polymers.” Electrochim. Acta, 48 (9) 1257–1262 (2003)
Tan, CK, Blackwood, DJ, “Corrosion Protection by Multilayered Conducting Polymer Coatings.” Corros. Sci., 45 (3) 545–557 (2003)
Annibaldi, V, Rooney, AD, Breslin, CB, “Corrosion Protection of Copper Using Polypyrrole Electrosynthesised from a Salicylate Solution.” Corros. Sci., 59 179–185 (2012)
Gonçalves, GS, Baldissera, AF, Rodrigues, LF, Jr, Martini, EMA, Ferreira, CA, “Alkyd Coatings Containing Polyanilines for Corrosion Protection of Mild Steel.” Synth. Met., 161 (3–4) 313–323 (2011)
Palraj, S, Selvaraj, M, Vidhya, M, Rajagopal, G, “Synthesis and Characterization of Epoxy–Silicone–Polythiophene Interpenetrating Polymer Network for Corrosion Protection of Steel.” Prog. Org. Coat., 75 (4) 356–363 (2012)
Deshpande, P, Jadhav, N, Gelling, V, Sazou, D, “Conducting Polymers for Corrosion Protection: A Review.” J. Coat. Technol. Res., 11 (4) 473–494 (2014)
Lim, CT, Goh, JCH, 13th International Conference on Biomedical Engineering: ICBME 2008, December, 3–6, 2008, Singapore. Springer London, Limited, 2009
Yan, M, Vetter, CA, Gelling, VJ, “Electrochemical Investigations of Polypyrrole Aluminum Flake Coupling.” Electrochim. Acta, 55 (20) 5576–5583 (2010)
Trueba, M, Trasatti, SP, “Characterization and Corrosion Performance of Poly(pyrrole-siloxane) Films on Commercial Al Alloys.” J. Appl. Electrochem., 39 (11) 2061–2072 (2009)
Ashraf, SA, Chen, F, Too, CO, Wallace, GG, “Bulk Electropolymerization of Alkylpyrroles.” Polymer, 37 (13) 2811–2819 (1996)
Gelling, VJ, Wiest, MM, Tallman, DE, Bierwagen, GP, Wallace, GG, “Electroactive-Conducting Polymers for Corrosion Control: 4. Studies of Poly(3-octyl pyrrole) and Poly(3-octadecyl pyrrole) on Aluminum 2024-T3 Alloy.” Prog. Org. Coat., 43 (1–3) 149–157 (2001)
Peres, RCD, Pernaut, JM, De Paoli, M-A, “Properties of Poly(pyrrole) Films Electrochemically Synthesized in the Presence of Surfactants.” Synth. Met., 28 (1–2) 59–64 (1989)
Song, M-K, Kim, Y-T, Kim, B-S, Kim, J, Char, K, Rhee, H-W, “Synthesis and Characterization of Soluble Polypyrrole Doped with Alkylbenzenesulfonic Acids.” Synth. Met., 141 (3) 315–319 (2004)
Qi, X, Vetter, C, Harper, AC, Gelling, VJ, “Electrochemical Investigations into Polypyrrole/Aluminum Flake Pigmented Coatings.” Prog. Org. Coat., 63 (3) 345–351 (2008)
Jadhav, N, Vetter, CA, Gelling, VJ, “The Effect of Polymer Morphology on the Performance of a Corrosion Inhibiting Polypyrrole/Aluminum Flake Composite Pigment.” Electrochim. Acta, 102 28–43 (2013)
Jadhav, N, Gelling, VJ, “Synthesis and Characterization of Micaceous Iron Oxide/Polypyrrole Composite Pigments and Their Application for Corrosion Protection of Cold Rolled Steel.” Corrosion, 70 464–474 (2013)
Jadhav, N, Vetter, CA, Gelling, VJ, “Characterization and Electrochemical Investigations of Polypyrrole/Aluminum Flake Composite Pigments on AA 2024-T3 Substrate.” ECS Trans., 41 (15) 75–89 (2012)
Sabouri, M, Shahrabi, T, Farid, HR, Hosseini, MG, “Polypyrrole and Polypyrrole–Tungstate Electropolymerization Coatings on Carbon Steel and Evaluating Their Corrosion Protection Performance Via Electrochemical Impedance Spectroscopy.” Prog. Org. Coat., 64 (4) 429–434 (2009)
Sabouri, M, Shahrabi, T, Hosseini, MG, “Improving Corrosion Protection Performance of Polypyrrole Coating by Tungstate Ion Dopants.” Russ. J. Electrochem., 43 (12) 1390–1397 (2007)
Iannuzzi, M, Frankel, GS, “Mechanisms of Corrosion Inhibition of AA2024-T3 by Vanadates.” Corros. Sci., 49 (5) 2371–2391 (2007)
Mahmoudian, MR, Basirun, WJ, Alias, Y, “Synthesis and Characterization of Poly(N-methylpyrrole)/TiO2 Composites on Steel.” Appl. Surf. Sci., 257 (8) 3702–3708 (2011)
Akbarinezhad, E, Ebrahimi, M, Sharif, F, Attar, MM, Faridi, HR, “Synthesis and Evaluating Corrosion Protection Effects of Emeraldine Base PAni/Clay Nanocomposite as a Barrier Pigment in Zinc-Rich Ethyl Silicate Primer.” Prog. Org. Coat., 70 (1) 39–44 (2011)
Hosseini, MG, Jafari, M, Najjar, R, “Effect of Polyaniline–Montmorillonite Nanocomposite Powders Addition on Corrosion Performance of Epoxy Coatings on Al 5000.” Surf. Coat. Technol., 206 (2–3) 280–286 (2011)
Hosseini, MG, Bagheri, R, Najjar, R, “Electropolymerization of Polypyrrole and Polypyrrole–ZnO Nanocomposites on Mild Steel and Its Corrosion Protection Performance.” J. Appl. Polym. Sci., 121 (6) 3159–3166 (2011)
Ioniţă, M, Prună, A, “Polypyrrole/Carbon Nanotube Composites: Molecular Modeling and Experimental Investigation as Anti-corrosive Coating.” Prog. Org. Coat., 72 (4) 647–652 (2011)
Yan, M, Tallman, DE, Bierwagen, GP, “Role of Oxygen in the Galvanic Interaction Between Polypyrrole and Aluminum Alloy.” Electrochim. Acta, 54 (2) 220–227 (2008)
Rizzi, M, Trueba, M, Trasatti, SP, “Polypyrrole Films on Al Alloys: The Role of Structural Changes on Protection Performance.” Synth. Met., 161 (1–2) 23–31 (2011)
Nguyen Thi Le, H, Garcia, B, Deslouis, C, Le Xuan, Q, “Corrosion Protection and Conducting Polymers: Polypyrrole Films on Iron.” Electrochim. Acta, 46 (26–27) 4259–4272 (2001)
Lu, W-K, Elsenbaumer, RL, Wessling, B, “Corrosion Protection of Mild Steel by Coatings Containing Polyaniline.” Synth. Met., 71 (1–3) 2163–2166 (1995)
Tian, B, Zerbi, G, “Lattice-Dynamics and Vibrational-Spectra of Polypyrrole.” J. Chem. Phys., 92 (6) 3886–3891 (1990)
Kasisomayajula, SV, Qi, XN, Vetter, C, Croes, K, Pavlacky, D, Gelling, VJ, “A Structural and Morphological Comparative Study Between Chemically Synthesized and Photopolymerized Poly(pyrrole).” J. Coat. Technol. Res., 7 (2) 145–158 (2010)
Liang, W, Lei, J, Martin, CR, “Effect of Synthesis Temperature on the Structure, Doping Level and Charge-Transport Properties of Polypyrrole.” Synth. Met., 52 (2) 227–239 (1992)
Omastová, M, Trchová, M, Kovářová, J, Stejskal, J, “Synthesis and Structural Study of Polypyrroles Prepared in the Presence of Surfactants.” Synth. Met., 138 (3) 447–455 (2003)
Tian, B, Zerbi, G, “Lattice Dynamics and Vibrational Spectra of Pristine and Doped Polypyrrole: Effective Conjugation Coordinate.” J. Chem. Phys., 92 (6) 3892–3898 (1990)
Saravanan, C, Shekhar, RC, Palaniappan, S, “Synthesis of Polypyrrole Using Benzoyl Peroxide as a Novel Oxidizing Agent.” Macromol. Chem. Phys., 207 (3) 342–348 (2006)
Socrates, G, Infrared and Raman Characteristic Group Frequencies: Tables and Charts. Wiley, New York, 2004
Kaynak, A, “Effect of Synthesis Parameters on the Surface Morphology of Conducting Polypyrrole Films.” Mater. Res. Bull., 32 (3) 271–285 (1997)
Maddison, DS, Unsworth, J, “Optimization of Synthesis Conditions of Polypyrrole from Aqueous Solutions.” Synth. Met., 30 (1) 47–55 (1989)
Unsworth, J, Innis, PC, Lunn, BA, Jin, Z, Norton, GP, “The Influence of Electrolyte pH on the Surface Morphology of Polypyrrole.” Synth. Met., 53 (1) 59–69 (1992)
Goel, S, Mazumdar, NA, Gupta, A, “Synthesis and Characterization of Polypyrrole Nanofibers with Different Dopants.” Polym. Adv. Technol., 21 (3) 205–210 (2010)
Warren, LF, Walker, JA, Anderson, DP, Rhodes, CG, Buckley, LJ, “A Study of Conducting Polymer Morphology: The Effect of Dopant Anions upon Order.” J. Electrochem. Soc., 136 (8) 2286–2295 (1989)
Yang, C, Liu, P, “Water-Dispersed Polypyrrole Nanoparticles Via Chemical Oxidative Polymerization in the Presence of a Functional Polyanion.” React. Funct. Polym., 70 (10) 726–731 (2010)
Díez, I, Tauer, K, Schulz, B, “Unusual Polymer Dispersions—Polypyrrole Suspensions Made of Rings, Frames, and Platelets.” Colloid Polym. Sci., 284 (12) 1431–1442 (2006)
Lu, Y, Pich, A, Adler, H-JP, “Synthesis and Characterization of Polypyrrole Dispersions Prepared with Different Dopants.” Macromol. Symp., 210 (1) 411–417 (2004)
Brédas, JL, Scott, JC, Yakushi, K, Street, GB, “Polarons and Bipolarons in Polypyrrole: Evolution of the Band Structure and Optical Spectrum upon Doing.” Phys. Rev. B, 30 (2) 1023–1025 (1984)
Bredas, JL, Street, GB, “Polarons, Bipolarons, and Solitons in Conducting Polymers.” Acc. Chem. Res., 18 (10) 309–315 (1985)
Kuwabata, S, Nakamura, J, Yoneyama, H, “The Effect of Basicity of Dopant Anions on the Conductivity of Polypyrrole Films.” J. Chem. Soc. Chem. Commun., 0 (12) 779–780 (1988)
Shakoor, A, Rizvi, TZ, “Synthesis and Characterization of Polypyrrole Dodecylbenzenesulfonate-Titanium Dioxide Nanocomposites.” J. Appl. Polym. Sci., 117 (2) 970–973 (2010)
Jianguo, L, Gaoping, G, Chuanwei, Y, “EIS Study of Corrosion Behaviour of Organic Coating/Dacromet Composite Systems.” Electrochim. Acta, 50 (16–17) 3320–3332 (2005)
Mansfeld, F, “Use of Electrochemical Impedance Spectroscopy for the Study of Corrosion Protection by Polymer Coatings.” J. Appl. Electrochem., 25 (3) 187–202 (1995)
Truong, VT, Lai, PK, Moore, BT, Muscat, RF, Russo, MS, “Corrosion Protection of Magnesium by Electroactive Polypyrrole/Paint Coatings.” Synth. Met., 110 (1) 7–15 (2000)
Iroh, JO, Su, W, “Corrosion Performance of Polypyrrole Coating Applied to Low Carbon Steel by an Electrochemical Process.” Electrochim. Acta, 46 (1) 15–24 (2000)
Rohwerder, M, Michalik, A, “Conducting Polymers for Corrosion Protection: What Makes the Difference Between Failure and Success?” Electrochim. Acta, 53 (3) 1300–1313 (2007)
Yan, MC, Tallman, DE, Rasmussen, SC, Bierwagen, GP, “Corrosion Control Coatings for Aluminum Alloys Based on Neutral and n-Doped Conjugated Polymers.” J. Electrochem. Soc., 156 (10) C360–C366 (2009)
Kinlen, PJ, Menon, V, Ding, Y, “A Mechanistic Investigation of Polyaniline Corrosion Protection Using the Scanning Reference Electrode Technique.” J. Electrochem. Soc., 146 (10) 3690–3695 (1999)
Kendig, M, Hon, M, Warren, L, “‘Smart’ Corrosion Inhibiting Coatings.” Prog. Org. Coat., 47 (3–4) 183–189 (2003)
Tallman, DE, Levine, KL, Siripirom, C, Gelling, VG, Bierwagen, GP, Croll, SG, “Nanocomposite of Polypyrrole and Alumina Nanoparticles as a Coating Filler for the Corrosion Protection of Aluminium Alloy 2024-T3.” Appl. Surf. Sci., 254 (17) 5452–5459 (2008)
Jensen, M, Tallman, D, “A LabVIEW-Based Virtual Instrument for Simulation and Analysis of SECM Approach Curves.” J. Solid State Electrochem., 17 (12) 2999–3003 (2013)
Jensen, M, Peterson, M, Jadhav, N, Gelling, VJ, “SECM Investigation of Corrosion Inhibition by Tungstate- and Vanadate-Doped Polypyrrole/Aluminum Flake Composite Coatings on AA2024-T3.” Prog. Org. Coat., 77 (12) 2116–2122 (2014)
Acknowledgments
The authors gratefully acknowledge the support of this research by US Army Research Laboratory under Grant Nos. W911NF-09-2-0014, W911NF-10-2-0082, and W911NF-11-2-0027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jadhav, N., Jensen, M.B. & Gelling, V. Tungstate and vanadate-doped polypyrrole/aluminum flake composite coatings for the corrosion protection of aluminum 2024-T3. J Coat Technol Res 12, 259–276 (2015). https://doi.org/10.1007/s11998-014-9633-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-014-9633-4