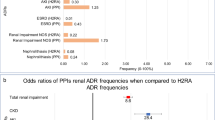
Opinion statement
Proton pump inhibitors (PPI) are among the safest class of drugs used by all care providers, including gastroenterologists. They are the mainstay in treatment of acid-related disease, in particular, gastroesophageal reflux disease. Without them, many patients would experience a major decrement in their quality of life. However, no drug is without side effects or adverse events. In the past decade, numerous reports, principally case control studies and meta-analyses, have raised questions about important adverse events related to the use of PPIs. This has affected not only physicians’ prescribing habits but patients’ concerns about using these medications, particularly long term. Several FDA warnings are listed including those related to long bone fractures, interaction with clopidogrel, enteric infections, and hypomagnesaemia. More recently, concerns regarding PPIs and cardiovascular events have resurfaced as have issues related to kidney disease and dementia. The methodology of these studies allows us to find an association with these events but does not provide us with sufficient evidence to determine causality. In general, the findings of the available studies do not fit with our clinical experience nor is the magnitude of the association sufficient to result in a major change in our practice. Nevertheless, the recent literature has resulted in our careful reevaluation of PPI use across both FDA indications and in general. This article will critically review the literature regarding potential PPI adverse events and attempt to place them in perspective for the practicing physician.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Katz P, Kahrilas P, Johnson D, et al. Daytime intragastric acid control: post hoc analyses of esomeprazole 20 mg and over-the-counter proton-pump inhibitors. Therap Adv Gastroenterol. 2015;8(6):322–30.
Wilder-Smith C, Backlund A, Eckerwall G, Lind T, Fjellman M, Rohss K. Effect of increasing esomeprazole and pantoprazole doses on acid control in patients with symptoms of gastro-oesophageal reflux disease: a randomized, dose-response study. Clin Drug Investig. 2008;28(6):333–43.
Savarino V, Mela GS, Zentilin P, et al. Comparison of 24-h control of gastric acidity by three different dosages of pantoprazole in patients with duodenal ulcer. Aliment Pharmacol Ther. 1998;12(12):1241–7.
Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas PJ. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42(6):649–63.
Kuipers EJ. Proton pump inhibitors and gastric neoplasia. Gut. 2006;55(9):1217–21.
Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108(7):2244–7.
Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32(8):821–7.
Frelinger III AL, Lee RD, Mulford DJ, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012;59(14):1304–11.
•• Cardoso RN, Benjo AM, DiNicolantonio JJ, et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis. Open Heart. 2015;2(1):e000248. An excellent meta-analysis outlning association of multiple cardiac issues and PPI use. Offers clarity among numerous studies.
Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909–17.
Hsu PI, Lai KH, Liu CP. Esomeprazole with clopidogrel reduces peptic ulcer recurrence, compared with clopidogrel alone, in patients with atherosclerosis. Gastroenterology. 2011;140(3):791–8.
Ghebremariam YT, LePendu P, Lee JC, et al. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation. 2013;128(8):845–53.
• Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10(6):e0124653. A pharmacovigilance, data mining study widely publicized by the lay press with complex methodology. Very difficult to completely accept methodology.
Ghebremariam YT, Cooke JP, Khan F, et al. Proton pump inhibitors and vascular function: a prospective cross-over pilot study. Vasc Med. 2010;20(4):309–16.
Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153(6):378–86.
Turkiewicz A, Vicente RP, Ohlsson H, Tyden P, Merlo J. Revising the link between proton-pump inhibitors and risk of acute myocardial infarction—a case-crossover analysis. Eur J Clin Pharmacol. 2015;71(1):125–9.
Shih CJ, Chen YT, Ou SM, Li SY, Chen TJ, Wang SJ. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int J Cardiol. 2014;177(1):292–7.
Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419–28.
•• Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410–4. Large database study from Germany offering an association between PPI use and dementia. Sample size is quite large understanding how dementia was coded not easy to do.
• Larazus B, Chen Y, Wilson F, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238–46. A case control study of two large databases. Shows consistent association of development of chronic kidney disease with PPIs. Size and consistency to results are strengths. Plausible etiology for CKD not clear from the study.
Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 27: 2016.
Termanini B, Gibril F, Sutliff VE, et al. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104(5):422–30.
O'Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778–81.
Epstein M, McGrath S, Law F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med. 2006;355(17):1834–6.
Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730–7.
• Attwood SE, Ell C, Galmiche JP, et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther. 2015;41(11):1162–74. A randomized prospective trial examing multiple outcomes. This study looked in part at laboratory data (magnesium, calcium, iron) showing no difference in medcial and surgical groups.
Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122(10):896–903.
den Elzen WP, Groeneveld Y, De RW, et al. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol Ther. 2008;27(6):491–7.
Hirschowitz BI, Worthington J, Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2008;27(11):1110–21.
Kopic S, Geibel JP. Gastric acid, calcium absorption, and their impact on bone health. Physiol Rev. 2013;93(1):189–268.
Mattsson JP, Vaananen K, Wallmark B, et al. Omeprazole and bafilomycin, two proton pump inhibitors: differentiation of their effects on gastric, kidney and bone H(+)-translocating ATPases. Biochim Biophys Acta. 1991;1065(2):261–8.
Wright MJ, Sullivan RR, Gaffney-Stomberg E, et al. Inhibiting gastric acid production does not affect intestinal calcium absorption in young, healthy individuals: a randomized, crossover, controlled clinical trial. J Bone Miner Res. 2010;25(10):2205–11.
Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947–53.
Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139(1):93–101.
Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170(9):765–71.
Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138(3):896–904.
Hansen KE, Jones AN, Lindstrom MJ, et al. Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res. 2010;25(12):2786–95.
Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106(7):1209–18. quiz 1219
Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102(9):2047–56.
Doorduyn Y, Van Den Brandhof WE, Van Duynhoven YT, et al. Risk factors for Salmonella enteritidis and typhimurium (DT104 and non-DT104) infections in the Netherlands: predominant roles for raw eggs in enteritidis and sandboxes in typhimurium infections. Epidemiol Infect. 2006;134(3):617–26.
Freeman R, Dabrera G, Lane C, et al. Association between use of proton pump inhibitors and non-typhoidal salmonellosis identified following investigation into an outbreak of Salmonella mikawasima in the UK. Epidemiol Infect. 2013;2015:1–8.
Doorduyn Y, Van Den Brandhof WE, Van Duynhoven YT, et al. Risk factors for indigenous Campylobacter jejuni and Campylobacter coli infections in the Netherlands: a case-control study. Epidemiol Infect. 2010;138(10):1391–404.
DuPont HL, Formal SB, Hornick RB, et al. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971;285(1):1–9.
Bavishi C, DuPont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34(11–12):1269–81.
Diemert DJ. Prevention and self-treatment of traveler’s diarrhea. Clin Microbiol Rev. 2006;19(3):583–94.
Juckett G. Prevention and treatment of traveler’s diarrhea. Am Fam Physician. 1999;60(1):119–24.
Schwille-Kiuntke J, Mazurak N, Enck P. Systematic review with meta-analysis: post-infectious irritable bowel syndrome after travellers’ diarrhoea. Aliment Pharmacol Ther. 2015;41(11):1029–37.
Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29(6):626–34.
Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001–10.
Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381–90.
FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). Food and Drug Administration.
Further reading
Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother. 2007;51(8):2883–7.
Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis study (CaMos). Am J Gastroenterol. 2012;107(9):1361–9.
Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
Freedberg DE, Toussaint NC, Chen SP, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology. 2015;149(4):883–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Felice Schnoll-Sussman declares no potential conflicts of interest. Philip O. Katz is a consultant for Pfizer Consumer Health and Torax and a speaker for Medtronic.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Esophagus
Rights and permissions
About this article
Cite this article
Schnoll-Sussman, F., Katz, P.O. Clinical Implications of Emerging Data on the Safety of Proton Pump Inhibitors. Curr Treat Options Gastro 15, 1–9 (2017). https://doi.org/10.1007/s11938-017-0115-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-017-0115-5