Abstract
Mainly due to the advent of next-generation sequencing (NGS), the field of genetics of dystonia has rapidly grown in recent years, which led to the discovery of a number of novel dystonia genes and the development of a new classification and nomenclature for inherited dystonias. In addition, new findings from both in vivo and in vitro studies have been published on the role of previously known dystonia genes, extending our understanding of the pathophysiology of dystonia. We here review the current knowledge and recent findings in the known genes for isolated dystonia TOR1A, THAP1, and GNAL as well as for the combined dystonias due to mutations in GCH1, ATP1A3, and SGCE. We present confirmatory evidence for a role of dystonia genes that had not yet been unequivocally established including PRKRA, TUBB4A, ANO3, and TAF1. We finally discuss selected novel genes for dystonia such as KMT2B and VAC14 along with the challenges for gene identification in the NGS era and the translational importance of dystonia genetics in clinical practice.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Oppenheim H. Ueber eigenenartige Krampfkrankheit des kindlichen und jugendlichen Alters (Dysbasia lordotica progressiva, Dystonia Musculorum Deformans). Neurol Centrabl. 1911;30:1090.
• C. Klein, S. Fahn, Translation of Oppenheim’s 1911 paper on dystonia, Mov Disord 28 (2013) 851-862. This is a translation and commentary of Oppenheim’s landmark paper coining the term dystonia and most likely describing the first cases of generalized dystonia due to mutations in Tor1A (formerly DYT1) and suspecting a genetic etiology.
Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov Disord. 2012;27:1789–96.
• Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–73. This is an important paper that revises the definition and classification of dystonia based on a consensus outcome of an international expert panel. The new classification contains two axes: clinical characteristics and etiology. The latter one replaces the terms “primary” and “dystonia-plus” dystonia with “isolated” and “combined” dystonia, respectively.
Wang L, Chen Y, Hu B, Hu X. Late-onset primary dystonia in Zhejiang province of China: a service-based epidemiological study. Neurol Sci. 2016;37:111–6.
L. Williams, E. McGovern, O. Kimmich, A. Molloy, I. Beiser, J.S. Butler, F. Molloy, P. Logan, D.G. Healy, T. Lynch, R. Walsh, L. Cassidy, P. Moriarty, H. Moore, T. McSwiney, C. Walsh, S. O’Riordan, M. Hutchinson, Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland, Eur J Neurol (2016).
Molloy A, Williams L, Kimmich O, Butler JS, Beiser I, McGovern E, et al. Sun exposure is an environmental factor for the development of blepharospasm. J Neurol Neurosurg Psychiatry. 2016;87:420–4.
Schmidt A, Jabusch HC, Altenmuller E, Hagenah J, Bruggemann N, Lohmann K, et al. Etiology of musician’s dystonia: familial or environmental? Neurology. 2009;72:1248–54.
Groen JL, Kallen MC, van de Warrenburg BP, Speelman JD, van Hilten JJ, Aramideh M, et al. Phenotypes and genetic architecture of focal primary torsion dystonia. J Neurol Neurosurg Psychiatry. 2012;83:1006–11.
van Egmond ME, Kuiper A, Eggink H, Sinke RJ, Brouwer OF, Verschuuren-Bemelmans CC, et al. Dystonia in children and adolescents: a systematic review and a new diagnostic algorithm. J Neurol Neurosurg Psychiatry. 2015;86:774–81.
• M. Zech, S. Boesch, A. Jochim, S. Weber, T. Meindl, B. Schormair, T. Wieland, C. Lunetta, V. Sansone, M. Messner, J. Mueller, A. Ceballos-Baumann, T.M. Strom, R. Colombo, W. Poewe, B. Haslinger, J. Winkelmann, Clinical exome sequencing in early-onset generalized dystonia and large-scale resequencing follow-up, Mov Disord (2016). This is a relevant paper in two respects: first, it uses exome sequencing to detect the cause of dystonia in a small group of patients underlining the heterogeneity of dystonia. Second, it described the first de novo mutation in ANO3 providing increasing evidence for a pathogenic role of mutations in this gene.
•• Marras C, Lang A, van de Warrenburg BP, Sue CM, Tabrizi SJ, Bertram L, et al. Nomenclature of genetic movement disorders: recommendations of the international Parkinson and movement disorder society task force. Mov Disord. 2016;31:436–57. This paper is of major importance since it suggests a new nomenclature system for genetic movement disorders based on extensive considerations and discussions among an expert panel and members of the Movement Disorder Society.
Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–8.
Marras C, Lohmann K, Lang A, Klein C. Fixing the broken system of genetic locus symbols: Parkinson disease and dystonia as examples. Neurology. 2012;78:1016–24.
Domingo A, Erro R, Lohmann K. Novel dystonia genes: clues on disease mechanisms and the complexities of high-throughput sequencing. Mov Disord. 2016;31:471–7.
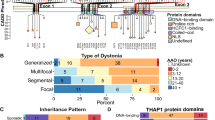
Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–8.
Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45:88–92.
Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–42.
de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, et al. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–75.
Zimprich A, Grabowski M, Asmus F, Naumann M, Berg D, Bertram M, et al. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet. 2001;29:66–9.
LeDoux MS. The genetics of dystonia. Adv Genet. 2012;79:35–85.
Bressman SB, Sabatti C, Raymond D, de Leon D, Klein C, Kramer PL, et al. The DYT1 phenotype and guidelines for diagnostic testing. Neurology. 2000;54:1746–52.
Dobricic V, Kresojevic N, Zarkovic M, Tomic A, Marjanovic A, Westenberger A, et al. Phenotype of non-c.907_909delGAG mutations in TOR1A: DYT1 dystonia revisited. Parkinsonism Relat Disord. 2015;21:1256–9.
Vulinovic F, Lohmann K, Rakovic A, Capetian P, Alvarez-Fischer D, Schmidt A, et al. Unraveling cellular phenotypes of novel TorsinA/TOR1A mutations. Hum Mutat. 2014;35:1114–22.
Zech M, Jochim A, Boesch S, Weber S, Meindl T, Peters A, et al. Systematic TOR1A non-c.907_909delGAG variant analysis in isolated dystonia and controls. Parkinsonism Relat Disord. 2016;31:119–23.
Hettich J, Ryan SD, de Souza ON, Saraiva Macedo Timmers LF, Tsai S, Atai NA, et al. Biochemical and cellular analysis of human variants of the DYT1 dystonia protein, TorsinA/TOR1A. Hum Mutat. 2014;35:1101–13.
Kabakci K, Hedrich K, Leung JC, Mitterer M, Vieregge P, Lencer R, et al. Mutations in DYT1: extension of the phenotypic and mutational spectrum. Neurology. 2004;62:395–400.
• Brüggemann N, Kühn A, Schneider SA, Kamm C, Wolters A, Krause P, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology. 2015;84:895–903. This paper provides a translational aspect on the partially differential clinical outcome after deep brain stimulation in carriers of mutations in different dystonia genes.
Goodchild RE, Dauer WT. Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation. Proc Natl Acad Sci USA. 2004;101:847–52.
B.A. Sosa, F.E. Demircioglu, J.Z. Chen, J. Ingram, H.L. Ploegh, T.U. Schwartz, How lamina-associated polypeptide 1 (LAP1) activates Torsin, Elife 3 (2014) e03239.
Naismith TV, Dalal S, Hanson PI. Interaction of torsinA with its major binding partners is impaired by the dystonia-associated DeltaGAG deletion. J Biol Chem. 2009;284:27866–74.
F.E. Demircioglu, B.A. Sosa, J. Ingram, H.L. Ploegh, T.U. Schwartz, Structures of TorsinA and its disease-mutant complexed with an activator reveal the molecular basis for primary dystonia, Elife 5 (2016).
•• Grillet M, Dominguez Gonzalez B, Sicart A, Pottler M, Cascalho A, Billion K, et al. Torsins are essential regulators of cellular lipid metabolism. Dev Cell. 2016;38:235–47. This is a very important study that links Torsin mutations to dysregulated cellular lipid metabolism and thus suggests a novel disease mechanism in dystonia.
• V.G. Shakkottai, A. Batla, K. Bhatia, W.T. Dauer, C. Dresel, M. Niethammer, D. Eidelberg, R.S. Raike, Y. Smith, H.A. Jinnah, E.J. Hess, S. Meunier, M. Hallett, R. Fremont, K. Khodakhah, M.S. LeDoux, T. Popa, C. Gallea, S. Lehericy, A.C. Bostan, P.L. Strick, Current opinions and areas of consensus on the role of the cerebellum in dystonia, Cerebellum (2016). This is an important review summarizing and discussing data on the neuroanatomical site of origin of dystonia. It illustrates that the cerebellum plays a role in the pathophysiology of dystonia, but it is probably neither the primary nor sole relevant neuroanatomical structure.
E. Premi, M. Diano, S. Gazzina, F. Cauda, V. Gualeni, M. Tinazzi, M. Fiorio, P. Liberini, C. Lazzarini, S. Archetti, G. Biasotto, M. Turla, V. Bertasi, M. Cotelli, R. Gasparotti, A. Padovani, B. Borroni, Functional connectivity networks in asymptomatic and symptomatic DYT1 carriers, Mov Disord (2016).
Vanni V, Puglisi F, Bonsi P, Ponterio G, Maltese M, Pisani A, et al. Cerebellar synaptogenesis is compromised in mouse models of DYT1 dystonia. Exp Neurol. 2015;271:457–67.
Blanchard A, Ea V, Roubertie A, Martin M, Coquart C, Claustres M, et al. DYT6 dystonia: review of the literature and creation of the UMD Locus-Specific Database (LSDB) for mutations in the THAP1 gene. Hum Mutat. 2011;32:1213–24.
Lohmann K, Uflacker N, Erogullari A, Lohnau T, Winkler S, Dendorfer A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet. 2012;20:171–5.
Bressman SB, Raymond D, Fuchs T, Heiman GA, Ozelius LJ, Saunders-Pullman R. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–6.
Paudel R, Li A, Hardy J, Bhatia KP, Houlden H, Holton J. DYT6 dystonia: a neuropathological study. Neurodegener Dis. 2016;16:273–8.
Krause P, Bruggemann N, Volzmann S, Horn A, Kupsch A, Schneider GH, et al. Long-term effect on dystonia after pallidal deep brain stimulation (DBS) in three members of a family with a THAP1 mutation. J Neurol. 2015;262:2739–44.
Ortiz-Virumbrales M, Ruiz M, Hone E, Dolios G, Wang R, Morant A, et al. Dystonia type 6 gene product Thap1: identification of a 50 kDa DNA-binding species in neuronal nuclear fractions. Acta Neuropathol Commun. 2014;2:139.
Gavarini S, Cayrol C, Fuchs T, Lyons N, Ehrlich ME, Girard JP, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Ann Neurol. 2010;68:549–53.
Kaiser FJ, Osmanoric A, Rakovic A, Erogullari A, Uflacker N, Braunholz D, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1. Ann Neurol. 2010;68:554–9. DYT6.
LeDoux MS, Vemula SR, Xiao J, Thompson MM, Perlmutter JS, Wright LJ, et al. Clinical and genetic features of cervical dystonia in a large multicenter cohort. Neurol Genet. 2016;2:e69.
Vemula SR, Puschmann A, Xiao J, Zhao Y, Rudzinska M, Frei KP, et al. Role of Galpha(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet. 2013;22:2510–9.
• Masuho I, Fang M, Geng C, Zhang J, Jiang H, Ozgul RK, et al. Homozygous GNAL mutation associated with familial childhood-onset generalized dystonia. Neurol Genet. 2016;2:e78. This is an interesting case report on a family with homozygous mutations in an actually dominantly inherited disorder. The data are not only descriptive on the phenotypic level but are accompanied by detailed functional analysis of the mutation underlining the importance of functional studies.
Opladen T, Hoffmann G, Horster F, Hinz AB, Neidhardt K, Klein C, et al. Clinical and biochemical characterization of patients with early infantile onset of autosomal recessive GTP cyclohydrolase I deficiency without hyperphenylalaninemia. Mov Disord. 2011;26:157–61.
Kumar KR, Lohmann K, Masuho I, Miyamoto R, Ferbert A, Lohnau T, et al. Mutations in GNAL: a novel cause of craniocervical dystonia. JAMA Neurol. 2014;71:490–4.
• Chen DH, Meneret A, Friedman JR, Korvatska O, Gad A, Bonkowski ES, et al. ADCY5-related dyskinesia: broader spectrum and genotype-phenotype correlations. Neurology. 2015;85:2026–35. This study reports many cases of ADCY5-related dyskinesia with a wide range of hyperkinetic abnormal movements.
V. Tadic, M. Kasten, N. Bruggemann, S. Stiller, J. Hagenah, C. Klein, Dopa-responsive dystonia revisited: diagnostic delay, residual signs, and nonmotor signs, Arch Neurol (2012) 1-5.
Lewthwaite AJ, Lambert TD, Rolfe EB, Olgiati S, Quadri M, Simons EJ, et al. Novel GCH1 variant in dopa-responsive dystonia and Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:394–7.
Mencacci NE, Isaias IU, Reich MM, Ganos C, Plagnol V, Polke JM, et al. Parkinson’s disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137:2480–92.
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989–93.
Hagenah J, Saunders-Pullman R, Hedrich K, Kabakci K, Habermann K, Wiegers K, et al. High mutation rate in dopa-responsive dystonia: detection with comprehensive GCHI screening. Neurology. 2005;64:908–11.
Douglas G, Hale AB, Crabtree MJ, Ryan BJ, Hansler A, Watschinger K, et al. A requirement for Gch1 and tetrahydrobiopterin in embryonic development. Dev Biol. 2015;399:129–38.
Brüggemann N, Spiegler J, Hellenbroich Y, Opladen T, Schneider SA, Stephani U, et al. Beneficial prenatal levodopa therapy in autosomal recessive guanosine triphosphate cyclohydrolase 1 deficiency. Arch Neurol. 2012;69:1071–5.
Brashear A, Dobyns WB, de Carvalho Aguiar P, Borg M, Frijns CJ, Gollamudi S, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–35.
• Sweney MT, Newcomb TM, Swoboda KJ. The expanding spectrum of neurological phenotypes in children with ATP1A3 mutations, alternating hemiplegia of childhood, rapid-onset dystonia-parkinsonism, CAPOS and beyond. Pediatr Neurol. 2015;52:56–64. This important article illustrates the broad phenotypic spectrum of mutations in a single gene, i.e., ATP1A3.
Holm TH, Isaksen TJ, Glerup S, Heuck A, Bottger P, Fuchtbauer EM, et al. Cognitive deficits caused by a disease-mutation in the alpha3 Na(+)/K(+)-ATPase isoform. Sci Rep. 2016;6:31972.
M. Hully, J. Ropars, L. Hubert, N. Boddaert, M. Rio, M. Bernardelli, I. Desguerre, V. Cormier-Daire, A. Munnich, P. de Lonlay, L. Reilly, C. Besmond, N. Bahi-Buisson, Mosaicism in ATP1A3-related disorders: not just a theoretical risk, Neurogenetics (2016).
Grünewald A, Djarmati A, Lohmann-Hedrich K, Farrell K, Zeller JA, Allert N, et al. Myoclonus-dystonia: significance of large SGCE deletions. Hum Mutat. 2008;29:331–2.
Peall KJ, Kurian MA, Wardle M, Waite AJ, Hedderly T, Lin JP, et al. SGCE and myoclonus dystonia: motor characteristics, diagnostic criteria and clinical predictors of genotype. J Neurol. 2014;261:2296–304.
Peall KJ, Dijk JM, Saunders-Pullman R, Dreissen YE, van Loon I, Cath D, et al. Psychiatric disorders, myoclonus dystonia and SGCE: an international study. Ann Clin Transl Neurol. 2016;3:4–11.
Müller B, Hedrich K, Kock N, Dragasevic N, Svetel M, Garrels J, et al. Evidence that paternal expression of the epsilon-sarcoglycan gene accounts for reduced penetrance in myoclonus-dystonia. Am J Hum Genet. 2002;71:1303–11.
Hack AA, Groh ME, McNally EM. Sarcoglycans in muscular dystrophy. Microsc Res Tech. 2000;48:167–80.
• Boulay AC, Saubamea B, Cisternino S, Mignon V, Mazeraud A, Jourdren L, et al. The sarcoglycan complex is expressed in the cerebrovascular system and is specifically regulated by astroglial Cx30 channels. Front Cell Neurosci. 2015;9:9. This functional study highlights a potential disease mechanism of mutations in the SGCE gene by linking the sarcoglycan complex to the cerebrovascular system.
•• A. Keller, A. Westenberger, M.J. Sobrido, M. Garcia-Murias, A. Domingo, R.L. Sears, R.R. Lemos, A. Ordonez-Ugalde, G. Nicolas, J.E. da Cunha, E.J. Rushing, M. Hugelshofer, M.C. Wurnig, A. Kaech, R. Reimann, K. Lohmann, V. Dobricic, A. Carracedo, I. Petrovic, J.M. Miyasaki, I. Abakumova, M.A. Mae, E. Raschperger, M. Zatz, K. Zschiedrich, J. Klepper, E. Spiteri, J.M. Prieto, I. Navas, M. Preuss, C. Dering, M. Jankovic, M. Paucar, P. Svenningsson, K. Saliminejad, H.R. Khorshid, I. Novakovic, A. Aguzzi, A. Boss, I. Le Ber, G. Defer, D. Hannequin, V.S. Kostic, D. Campion, D.H. Geschwind, G. Coppola, C. Betsholtz, C. Klein, J.R. Oliveira, Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice, Nat Genet (2013). This comprehensive study elucidated a frequent cause of primary familial brain calcification by identification of mutations in the PDGFB gene. It not only reported the cause of the disease in several multiplex families but also demonstrated corresponding brain calcifications in mouse models at different ages and linked this complex form of dystonia to dysfunction of the blood brain barrier.
Nicolas G, Pottier C, Maltete D, Coutant S, Rovelet-Lecrux A, Legallic S, et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology. 2013;80:181–7.
Westenberger A, Klein C. The genetics of primary familial brain calcifications. Curr Neurol Neurosci Rep. 2014;14:490.
Camargos S, Scholz S, Simon-Sanchez J, Paisan-Ruiz C, Lewis P, Hernandez D, et al. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–15.
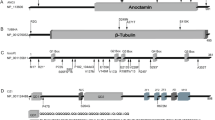
Hersheson J, Mencacci NE, Davis M, Macdonald N, Trabzuni D, Ryten M, et al. Mutations in the autoregulatory domain of beta-tubulin 4a cause hereditary dystonia. Ann Neurol. 2013;73:546–53.
Lohmann K, Wilcox RA, Winkler S, Ramirez A, Rakovic A, Park JS, et al. Whispering dysphonia (DYT4 dystonia) is caused by a mutation in the TUBB4 gene. Ann Neurol. 2013;73:537–45.
Charlesworth G, Plagnol V, Holmstrom KM, Bras J, Sheerin UM, Preza E, et al. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am J Hum Genet. 2012;91:1041–50.
Zech M, Gross N, Jochim A, Castrop F, Kaffe M, Dresel C, et al. Rare sequence variants in ANO3 and GNAL in a primary torsion dystonia series and controls. Mov Disord. 2014;29:143–7.
Nolte D, Niemann S, Muller U. Specific sequence changes in multiple transcript system DYT3 are associated with X-linked dystonia parkinsonism. Proc Natl Acad Sci USA. 2003;100:10347–52.
• Quadri M, Olgiati S, Sensi M, Gualandi F, Groppo E, Rispoli V, et al. PRKRA mutation causing early-onset generalized dystonia-parkinsonism (DYT16) in an Italian family. Mov Disord. 2016;31:765–7. This interesting paper confirms biallelic mutations in the PRKRA gene as a cause of dystonia-parkinsonism and demonstrates a founder effect.
Wilcox RA, Winkler S, Lohmann K, Klein C. Whispering dysphonia in an Australian family (DYT4): a clinical and genetic reappraisal. Mov Disord. 2011;26:2404–8.
Zech M, Boesch S, Jochim A, Graf S, Lichtner P, Peters A, et al. Large-scale TUBB4A mutational screening in isolated dystonia and controls. Parkinsonism Relat Disord. 2015;21:1278–81.
Erro R, Hersheson J, Ganos C, Mencacci NE, Stamelou M, Batla A, et al. H-ABC syndrome and DYT4: variable expressivity or pleiotropy of TUBB4 mutations? Mov Disord. 2015;30:828–33.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Lee LV, Pascasio FM, Fuentes FD, Viterbo GH. Torsion dystonia in Panay. Philippines Adv Neurol. 1976;14:137–51.
Domingo A, Westenberger A, Lee LV, Braenne I, Liu T, Vater I, et al. New insights into the genetics of X-linked dystonia-parkinsonism. Eur J Hum Genet. 2015;23:1334–40. XDP, DYT3.
Domingo A, Amar D, Grutz K, Lee LV, Rosales R, Bruggemann N, et al. Evidence of TAF1 dysfunction in peripheral models of X-linked dystonia-parkinsonism. Cell Mol Life Sci. 2016;73:3205–15.
•• J.A. O’Rawe, Y. Wu, M.J. Dorfel, A.F. Rope, P.Y. Au, J.S. Parboosingh, S. Moon, M. Kousi, K. Kosma, C.S. Smith, M. Tzetis, J.L. Schuette, R.B. Hufnagel, C.E. Prada, F. Martinez, C. Orellana, J. Crain, A. Caro-Llopis, S. Oltra, S. Monfort, L.T. Jimenez-Barron, J. Swensen, S. Ellingwood, R. Smith, H. Fang, S. Ospina, S. Stegmann, N. Den Hollander, D. Mittelman, G. Highnam, R. Robison, E. Yang, L. Faivre, A. Roubertie, J.B. Riviere, K.G. Monaghan, K. Wang, E.E. Davis, N. Katsanis, V.M. Kalscheuer, E.H. Wang, K. Metcalfe, T. Kleefstra, A.M. Innes, S. Kitsiou-Tzeli, M. Rosello, C.E. Keegan, G.J. Lyon, TAF1 variants are associated with dysmorphic features, intellectual disability, and neurological manifestations, Am J Hum Genet 97 (2015) 922-932. This manuscript describes bona fide mutations in the TAF1 gene, the gene that is also dysregulated in X-linked dystonia-parkinsonism (XDP) due to several disease-specific changes.
Hagenah JM, Zuhlke C, Hellenbroich Y, Heide W, Klein C. Focal dystonia as a presenting sign of spinocerebellar ataxia 17. Mov Disord. 2004;19:217–20.
Chen YZ, Matsushita MM, Robertson P, Rieder M, Girirajan S, Antonacci F, et al. Autosomal dominant familial dyskinesia and facial myokymia: single exome sequencing identifies a mutation in adenylyl cyclase 5. Arch Neurol. 2012;69:630–5.
Carapito R, Paul N, Untrau M, Le Gentil M, Ott L, Alsaleh G, et al. A de novo ADCY5 mutation causes early-onset autosomal dominant chorea and dystonia. Mov Disord. 2015;30:423–7.
F.C. Chang, A. Westenberger, R.C. Dale, M. Smith, H.S. Pall, B. Perez-Duenas, P. Grattan-Smith, R.A. Ouvrier, N. Mahant, B.C. Hanna, M. Hunter, J.A. Lawson, C. Max, R. Sachdev, E. Meyer, D. Crimmins, D. Pryor, J.G. Morris, A. Munchau, D. Grozeva, K.J. Carss, L. Raymond, M.A. Kurian, C. Klein, V.S. Fung, Phenotypic insights into ADCY5-associated disease, Mov Disord (2016).
•• S. Petrovski, S. Kury, C.T. Myers, K. Anyane-Yeboa, B. Cogne, M. Bialer, F. Xia, P. Hemati, J. Riviello, M. Mehaffey, T. Besnard, E. Becraft, A. Wadley, A.R. Politi, S. Colombo, X. Zhu, Z. Ren, I. Andrews, T. Dudding-Byth, A.L. Schneider, G. Wallace, A.B. Rosen, S. Schelley, G.M. Enns, P. Corre, J. Dalton, S. Mercier, X. Latypova, S. Schmitt, E. Guzman, C. Moore, L. Bier, E.L. Heinzen, P. Karachunski, N. Shur, T. Grebe, A. Basinger, J.M. Nguyen, S. Bezieau, K. Wierenga, J.A. Bernstein, I.E. Scheffer, J.A. Rosenfeld, H.C. Mefford, B. Isidor, D.B. Goldstein, Germline De Novo Mutations in GNB1 Cause Severe Neurodevelopmental Disability, Hypotonia, and Seizures, Am J Hum Genet 98 (2016) 1001-1010. This nicely performed screening study elucidated mutations in the GNB1 gene as a rare but recurrent cause of a complex form of dystonia and highlights G protein-mediated signaling as one disease mechanism in dystonia and neurodevelopmental disability.
Steinrucke S, Lohmann K, Domingo A, Rolfs A, Baumer T, Spiegler J, et al. Novel GNB1 missense mutation in a patient with generalized dystonia, hypotonia, and intellectual disability. Neurol Genet. 2016;2:e106.
L.A. Menke, M. Engelen, M. Alders, V.J. Odekerken, F. Baas, J.M. Cobben, Recurrent GNAO1 mutations associated with developmental delay and a movement disorder, J Child Neurol (2016).
Nakamura K, Kodera H, Akita T, Shiina M, Kato M, Hoshino H, et al. De Novo mutations in GNAO1, encoding a Galphao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet. 2013;93:496–505.
Lenk GM, Szymanska K, Debska-Vielhaber G, Rydzanicz M, Walczak A, Bekiesinska-Figatowska M, et al. Biallelic mutations of VAC14 in pediatric-onset neurological disease. Am J Hum Genet. 2016;99:188–94.
S. Edvardson, G. Tian, H. Cullen, H. Vanyai, L. Ngo, S. Bhat, A. Aran, M. Daana, N. Da’amseh, B. Abu-Libdeh, N.J. Cowan, J. Heng, O. Elpeleg, Infantile neurodegenerative disorder associated with mutations in TBCD, an essential gene in the tubulin heterodimer assembly pathway, Hum Mol Genet (2016).
•• Zech M, Boesch S, Maier EM, Borggraefe I, Vill K, Laccone F, et al. Haploinsufficiency of KMT2B, encoding the lysine-specific histone methyltransferase 2B, results in early-onset generalized dystonia. Am J Hum Genet. 2016;99:1377–87. This paper represents one of the two studies elucidating KMT2B mutations as a relatively common cause of early-onset dystonia.
•• E. Meyer, K.J. Carss, J. Rankin, J.M. Nichols, D. Grozeva, A.P. Joseph, N.E. Mencacci, A. Papandreou, J. Ng, S. Barral, A. Ngoh, H. Ben-Pazi, M.A. Willemsen, D. Arkadir, A. Barnicoat, H. Bergman, S. Bhate, A. Boys, N. Darin, N. Foulds, N. Gutowski, A. Hills, H. Houlden, J.A. Hurst, Z. Israel, M. Kaminska, P. Limousin, D. Lumsden, S. McKee, S. Misra, S.S. Mohammed, V. Nakou, J. Nicolai, M. Nilsson, H. Pall, K.J. Peall, G.B. Peters, P. Prabhakar, M.S. Reuter, P. Rump, R. Segel, M. Sinnema, M. Smith, P. Turnpenny, S.M. White, D. Wieczorek, S. Wiethoff, B.T. Wilson, G. Winter, C. Wragg, S. Pope, S.J. Heales, D. Morrogh, A. Pittman, L.J. Carr, B. Perez-Duenas, J.P. Lin, A. Reis, W.A. Gahl, C. Toro, K.P. Bhatia, N.W. Wood, E.J. Kamsteeg, W.K. Chong, P. Gissen, M. Topf, R.C. Dale, J.R. Chubb, F.L. Raymond, M.A. Kurian, Mutations in the histone methyltransferase gene KMT2B cause complex early-onset dystonia, Nat Genet (2016). This paper represents one of the two studies elucidating KMT2B mutations as a relatively common cause of early-onset dystonia.
K. Lohmann, C. Klein, Next generation sequencing and the future of genetic diagnosis, Neurotherapeutics (2014).
Olgiati S, Quadri M, Bonifati V. Genetics of movement disorders in the next-generation sequencing era. Mov Disord. 2016;31:458–70.
Mencacci NE, R’Bibo L, Bandres-Ciga S, Carecchio M, Zorzi G, Nardocci N, et al. The CACNA1B R1389H variant is not associated with myoclonus-dystonia in a large European multicentric cohort. Hum Mol Genet. 2015;24:5326–9.
Lohmann K, Klein C. Genetics of dystonia: what’s known? what’s new? what’s next? Mov Disord. 2013;28:899–905.
Mok KY, Schneider SA, Trabzuni D, Stamelou M, Edwards M, Kasperaviciute D, et al. Genomewide association study in cervical dystonia demonstrates possible association with sodium leak channel. Mov Disord. 2014;29:245–51.
Lohmann K, Schmidt A, Schillert A, Winkler S, Albanese A, Baas F, et al. Genome-wide association study in musician’s dystonia: a risk variant at the arylsulfatase G locus? Mov Disord. 2014;29:921–7.
Veltman JA, Brunner HG. De novo mutations in human genetic disease. Nat Rev Genet. 2012;13:565–75.
Acknowledgments
Katja Lohmann is supported by the DFG (LO 1555/4-1 and LO 1555/3-2). Christine Klein is a recipient of a career development award from the Hermann and Lilly Schilling Foundation. Further, Katja Lohmann and Christine Klein are supported by the German Ministry of Education and Research (BMBF, DYSTRACT consortium, 01GM1514B) and a Research Unit of the DFG (FOR2488).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Katja Lohmann declares no conflict of interest.
Christine Klein has received personal fees from Centogene and from the Else Kroener Fresenius Foundation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Lohmann, K., Klein, C. Update on the Genetics of Dystonia. Curr Neurol Neurosci Rep 17, 26 (2017). https://doi.org/10.1007/s11910-017-0735-0
Published:
DOI: https://doi.org/10.1007/s11910-017-0735-0